Co-administration of α-GalCer analog and TLR4 agonist induces robust CD8(+) T-cell responses to PyCS protein and WT-1 antigen and activates memory-like effector NKT cells
- PMID: 27132023
- PMCID: PMC4940295
- DOI: 10.1016/j.clim.2016.04.014
Co-administration of α-GalCer analog and TLR4 agonist induces robust CD8(+) T-cell responses to PyCS protein and WT-1 antigen and activates memory-like effector NKT cells
Abstract
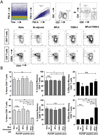
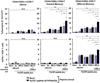
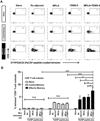
In the present study, the combined adjuvant effect of 7DW8-5, a potent α-GalCer-analog, and monophosphoryl lipid A (MPLA), a TLR4 agonist, on the induction of vaccine-induced CD8(+) T-cell responses and protective immunity was evaluated. Mice were immunized with peptides corresponding to the CD8(+) T-cell epitopes of a malaria antigen, a circumsporozoite protein of Plasmodium yoelii, and a tumor antigen, a Wilms Tumor antigen-1 (WT-1), together with 7DW8-5 and MPLA, as an adjuvant. These immunization regimens were able to induce higher levels of CD8(+) T-cell responses and, ultimately, enhanced levels of protection against malaria and tumor challenges compared to the levels induced by immunization with peptides mixed with 7DW8-5 or MPLA alone. Co-administration of 7DW8-5 and MPLA induces activation of memory-like effector natural killer T (NKT) cells, i.e. CD44(+)CD62L(-)NKT cells. Our study indicates that 7DW8-5 greatly enhances important synergistic pathways associated to memory immune responses when co-administered with MPLA, thus rendering this combination of adjuvants a novel vaccine adjuvant formulation.
Keywords: Adjuvant; CD1d; Cancer vaccine; Circumsporozoite protein; Glycolipid; Malaria vaccine; Memory-like effector NKT cells; NKT cells; TLR4; WT-1.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Colocalization of a CD1d-Binding Glycolipid with a Radiation-Attenuated Sporozoite Vaccine in Lymph Node-Resident Dendritic Cells for a Robust Adjuvant Effect.J Immunol. 2015 Sep 15;195(6):2710-21. doi: 10.4049/jimmunol.1403017. Epub 2015 Aug 7. J Immunol. 2015. PMID: 26254338 Free PMC article.
-
Co-localization of a CD1d-binding glycolipid with an adenovirus-based malaria vaccine for a potent adjuvant effect.Vaccine. 2017 May 31;35(24):3171-3177. doi: 10.1016/j.vaccine.2017.04.077. Epub 2017 May 5. Vaccine. 2017. PMID: 28483194 Free PMC article.
-
Identification of minimal CD8+ and CD4+ T cell epitopes in the Plasmodium yoelii hepatocyte erythrocyte protein 17kDa.Mol Immunol. 2007 Apr;44(11):3037-48. doi: 10.1016/j.molimm.2007.01.001. Epub 2007 Feb 15. Mol Immunol. 2007. PMID: 17303242
-
Innate Valpha14(+) natural killer T cells mature dendritic cells, leading to strong adaptive immunity.Immunol Rev. 2007 Dec;220:183-98. doi: 10.1111/j.1600-065X.2007.00561.x. Immunol Rev. 2007. PMID: 17979847 Review.
-
Immunological responses of neonates and infants to DNA vaccines.Methods Mol Med. 2006;127:239-51. doi: 10.1385/1-59745-168-1:239. Methods Mol Med. 2006. PMID: 16988458 Review.
Cited by
-
Novel Peptide-Based PD1 Immunomodulators Demonstrate Efficacy in Infectious Disease Vaccines and Therapeutics.Front Immunol. 2020 Mar 6;11:264. doi: 10.3389/fimmu.2020.00264. eCollection 2020. Front Immunol. 2020. PMID: 32210956 Free PMC article.
-
Malaria vaccines: a new era of prevention and control.Nat Rev Microbiol. 2024 Dec;22(12):756-772. doi: 10.1038/s41579-024-01065-7. Epub 2024 Jul 18. Nat Rev Microbiol. 2024. PMID: 39025972 Review.
-
Sex-Specific Differences in Cytokine Induction by the Glycolipid Adjuvant 7DW8-5 in Mice.Biomolecules. 2022 Dec 21;13(1):8. doi: 10.3390/biom13010008. Biomolecules. 2022. PMID: 36671393 Free PMC article.
-
Targeted Co-delivery of Tumor Antigen and α-Galactosylceramide to CD141+ Dendritic Cells Induces a Potent Tumor Antigen-Specific Human CD8+ T Cell Response in Human Immune System Mice.Front Immunol. 2020 Aug 18;11:2043. doi: 10.3389/fimmu.2020.02043. eCollection 2020. Front Immunol. 2020. PMID: 32973811 Free PMC article.
-
Conformationally Restricted Analogues of α-Galactosylceramide as Adjuvant in COVID-19 Subunit Vaccine.ACS Med Chem Lett. 2023 Nov 20;14(12):1647-1655. doi: 10.1021/acsmedchemlett.3c00154. eCollection 2023 Dec 14. ACS Med Chem Lett. 2023. PMID: 38116441 Free PMC article.
References
-
- Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what's in a name? Nat Rev Immunol. 2004;4:231–237. - PubMed
-
- Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. - PubMed
-
- Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–986. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

