Chronic myeloid leukemia: reminiscences and dreams
- PMID: 27132280
- PMCID: PMC5004358
- DOI: 10.3324/haematol.2015.139337
Chronic myeloid leukemia: reminiscences and dreams
Abstract
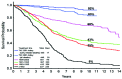
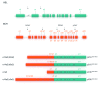
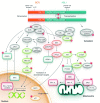
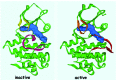
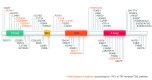
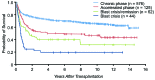
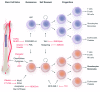
With the deaths of Janet Rowley and John Goldman in December 2013, the world lost two pioneers in the field of chronic myeloid leukemia. In 1973, Janet Rowley, unraveled the cytogenetic anatomy of the Philadelphia chromosome, which subsequently led to the identification of the BCR-ABL1 fusion gene and its principal pathogenetic role in the development of chronic myeloid leukemia. This work was also of major importance to support the idea that cytogenetic changes were drivers of leukemogenesis. John Goldman originally made seminal contributions to the use of autologous and allogeneic stem cell transplantation from the late 1970s onwards. Then, in collaboration with Brian Druker, he led efforts to develop ABL1 tyrosine kinase inhibitors for the treatment of patients with chronic myeloid leukemia in the late 1990s. He also led the global efforts to develop and harmonize methodology for molecular monitoring, and was an indefatigable organizer of international conferences. These conferences brought together clinicians and scientists, and accelerated the adoption of new therapies. The abundance of praise, tributes and testimonies expressed by many serve to illustrate the indelible impressions these two passionate and affable scholars made on so many people's lives. This tribute provides an outline of the remarkable story of chronic myeloid leukemia, and in writing it, it is clear that the historical triumph of biomedical science over this leukemia cannot be considered without appreciating the work of both Janet Rowley and John Goldman.
Copyright© Ferrata Storti Foundation.
Figures



References
-
- Ben-Neriah Y, Daley GQ, Mes-Masson AM, et al. The chronic myelogenous leukemia-specific P210 protein is the product of the bcr/abl hybrid gene. Science. 1986;233(4760):212–214. - PubMed
-
- Goldman JM, Melo JV. Targeting the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344(14):1084–1086. - PubMed
-
- Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2(5):561–566. - PubMed
-
- Druker BJ, Guilhot F, O’Brien SG, et al. Five-year Follow-up of Patients Receiving Imatinib for Chronic Myeloid Leukemia. N Engl J Med. 2006;355(23):2408–2417. - PubMed
-
- Kantarjian H, Cortes JE. Complete cytogenetic response, not deep molecular response, is associated with survival in chronic myeloid leukemia. J Clin Oncol. 2014;32(27):3077. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

