Mechanism and Role of SOX2 Repression in Seminoma: Relevance to Human Germline Specification
- PMID: 27132888
- PMCID: PMC4939754
- DOI: 10.1016/j.stemcr.2016.04.002
Mechanism and Role of SOX2 Repression in Seminoma: Relevance to Human Germline Specification
Abstract
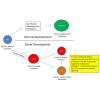
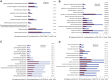
Human male germ cell tumors (GCTs) are derived from primordial germ cells (PGCs). The master pluripotency regulator and neuroectodermal lineage effector transcription factor SOX2 is repressed in PGCs and the seminoma (SEM) subset of GCTs. The mechanism of SOX2 repression and its significance to GC and GCT development currently are not understood. Here, we show that SOX2 repression in SEM-derived TCam-2 cells is mediated by the Polycomb repressive complex (PcG) and the repressive H3K27me3 chromatin mark that are enriched at its promoter. Furthermore, SOX2 repression in TCam-2 cells can be abrogated by recruitment of the constitutively expressed H3K27 demethylase UTX to the SOX2 promoter through retinoid signaling, leading to expression of neuronal and other lineage genes. SOX17 has been shown to initiate human PGC specification, with its target PRDM1 suppressing mesendodermal genes. Our results are consistent with a role for SOX2 repression in normal germline development by suppressing neuroectodermal genes.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Andrews P.W., Matin M.M., Bahrami A.R., Damjanov I., Gokhale P., Draper J.S. Embryonic stem (ES) cells and embryonal carcinoma (EC) cells: opposite sides of the same coin. Biochem. Soc. Trans. 2005;6:1526–1530. - PubMed
-
- Chaganti R.S., Houldsworth J. Genetics and biology of adult human male germ cell tumors. Cancer Res. 2000;60:1475–1482. - PubMed
-
- De Miguel M.P., Fuentes-Julián S., Alcaina Y. Pluripotent stem cells: origin, maintenance and induction. Stem Cell Rev. 2010;6:633–649. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

