Age-Dependent Pancreatic Gene Regulation Reveals Mechanisms Governing Human β Cell Function
- PMID: 27133132
- PMCID: PMC4864151
- DOI: 10.1016/j.cmet.2016.04.002
Age-Dependent Pancreatic Gene Regulation Reveals Mechanisms Governing Human β Cell Function
Abstract
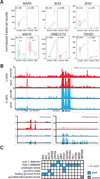
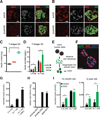
Intensive efforts are focused on identifying regulators of human pancreatic islet cell growth and maturation to accelerate development of therapies for diabetes. After birth, islet cell growth and function are dynamically regulated; however, establishing these age-dependent changes in humans has been challenging. Here, we describe a multimodal strategy for isolating pancreatic endocrine and exocrine cells from children and adults to identify age-dependent gene expression and chromatin changes on a genomic scale. These profiles revealed distinct proliferative and functional states of islet α cells or β cells and histone modifications underlying age-dependent gene expression changes. Expression of SIX2 and SIX3, transcription factors without prior known functions in the pancreas and linked to fasting hyperglycemia risk, increased with age specifically in human islet β cells. SIX2 and SIX3 were sufficient to enhance insulin content or secretion in immature β cells. Our work provides a unique resource to study human-specific regulators of islet cell maturation and function.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

