Promoting Neuronal Tolerance of Diabetic Stress: Modulating Molecular Chaperones
- PMID: 27133150
- PMCID: PMC4929789
- DOI: 10.1016/bs.irn.2016.03.001
Promoting Neuronal Tolerance of Diabetic Stress: Modulating Molecular Chaperones
Abstract
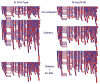
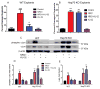
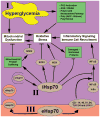
The etiology of diabetic peripheral neuropathy (DPN) involves an interrelated series of metabolic and vascular insults that ultimately contribute to sensory neuron degeneration. In the quest to pharmacologically manage DPN, small-molecule inhibitors have targeted proteins and pathways regarded as "diabetes specific" as well as others whose activity are altered in numerous disease states. These efforts have not yielded any significant therapies, due in part to the complicating issue that the biochemical contribution of these targets/pathways to the progression of DPN does not occur with temporal and/or biochemical uniformity between individuals. In a complex, chronic neurodegenerative disease such as DPN, it is increasingly appreciated that effective disease management may not necessarily require targeting a pathway or protein considered to contribute to disease progression. Alternatively, it may prove sufficiently beneficial to pharmacologically enhance the activity of endogenous cytoprotective pathways to aid neuronal tolerance to and recovery from glucotoxic stress. In pursuing this paradigm shift, we have shown that modulating the activity and expression of molecular chaperones such as heat shock protein 70 (Hsp70) may provide translational potential for the effective medical management of insensate DPN. Considerable evidence supports that modulating Hsp70 has beneficial effects in improving inflammation, oxidative stress, and glucose sensitivity. Given the emerging potential of modulating Hsp70 to manage DPN, the current review discusses efforts to characterize the cytoprotective effects of this protein and the benefits and limitations that may arise in drug development efforts that exploit its cytoprotective activity.
Keywords: Bioenergetics; Diabetic neuropathy; Heat shock proteins; Hsp70; Inflammation; Molecular chaperones; Novologues.
© 2016 Elsevier Inc. All rights reserved.
Figures
References
-
- Afolayan AJ, Teng RJ, Eis A, Rana U, Broniowska KA, Corbett JA, et al. Inducible Hsp70 regulates superoxide dismutase-2 and mitochondrial oxidative stress in the endothelial cells from developing lungs. American Journal of Physiology: Lung Cellular and Molecular Physiology. 2014;306:L351–360. - PMC - PubMed
-
- Agou F, Ye F, Goffinont S, Courtois G, Yamaoka S, Israel A, et al. Nemo trimerizes through its coiled-coil C-terminal domain. Journal of Biological Chemistry. 2002;277:17464–17475. - PubMed
-
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, et al. Hsp70 stimulates cytokine production through a CD14-dependent pathway, demonstrating its dual role as a chaperone and cytokine. Nature Medicine. 2000;6:435–442. - PubMed
-
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, et al. Novel signal transduction pathway utilized by extracellular Hsp70: Role of toll-like receptor (TLR) 2 and TLR4. Jornal of Biological Chemistry. 2002;277:15028–15034. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

