Cannabinoid Type 2 Receptors Mediate a Cell Type-Specific Plasticity in the Hippocampus
- PMID: 27133464
- PMCID: PMC5533103
- DOI: 10.1016/j.neuron.2016.03.034
Cannabinoid Type 2 Receptors Mediate a Cell Type-Specific Plasticity in the Hippocampus
Abstract
Endocannabinoids (eCBs) exert major control over neuronal activity by activating cannabinoid receptors (CBRs). The functionality of the eCB system is primarily ascribed to the well-documented retrograde activation of presynaptic CB1Rs. We find that action potential-driven eCB release leads to a long-lasting membrane potential hyperpolarization in hippocampal principal cells that is independent of CB1Rs. The hyperpolarization, which is specific to CA3 and CA2 pyramidal cells (PCs), depends on the activation of neuronal CB2Rs, as shown by a combined pharmacogenetic and immunohistochemical approach. Upon activation, they modulate the activity of the sodium-bicarbonate co-transporter, leading to a hyperpolarization of the neuron. CB2R activation occurred in a purely self-regulatory manner, robustly altered the input/output function of CA3 PCs, and modulated gamma oscillations in vivo. To conclude, we describe a cell type-specific plasticity mechanism in the hippocampus that provides evidence for the neuronal expression of CB2Rs and emphasizes their importance in basic neuronal transmission.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


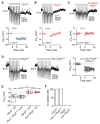
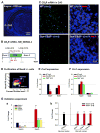

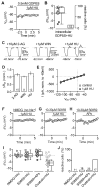
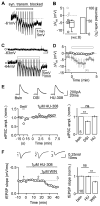

Comment in
-
A Central Move for CB2 Receptors.Neuron. 2016 May 18;90(4):670-1. doi: 10.1016/j.neuron.2016.05.012. Neuron. 2016. PMID: 27196970
References
-
- Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–316. - PubMed
-
- Baek JH, Darlington CL, Smith PF, Ashton JC. Antibody testing for brain immunohistochemistry: brain immunolabeling for the cannabinoid CB2 receptor. J Neurosci Methods. 2013;216:87–95. - PubMed
-
- Bouaboula M, Bianchini L, McKenzie FR, Pouyssegur J, Casellas P. Cannabinoid receptor CB1 activates the Na+/H+ exchanger NHE-1 isoform via Gi-mediated mitogen activated protein kinase signaling transduction pathways. FEBS Lett. 1999;449:61–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

