Physiological and pathological roles of the γ-secretase complex
- PMID: 27133790
- PMCID: PMC5436903
- DOI: 10.1016/j.brainresbull.2016.04.019
Physiological and pathological roles of the γ-secretase complex
Abstract
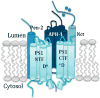
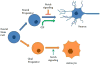
Gamma-secretase (GS) is an enzyme complex that cleaves numerous substrates, and it is best known for cleaving amyloid precursor protein (APP) to form amyloid-beta (Aβ) peptides. Aberrant cleavage of APP can lead to Alzheimer's disease, so much research has been done to better understand GS structure and function in hopes of developing therapeutics for Alzheimer's. Therefore, most of the attention in this field has been focused on developing modulators that reduce pathogenic forms of Aβ while leaving Notch and other GS substrates intact, but GS provides multiple avenues of modulation that could improve AD pathology. GS has complex regulation, through its essential subunits and other associated proteins, providing other targets for AD drugs. Therapeutics can also alter GS trafficking and thereby improve cognition, or move beyond Aβ entirely, effecting Notch and neural stem cells. GS also cleaves substrates that affect synaptic morphology and function, presenting another window by which GS modulation could improve AD pathology. Taken together, GS presents a unique cross road for neural processes and an ideal target for AD therapeutics.
Keywords: Alzheimer's disease; Amyloid-beta; Gamma-secretase.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
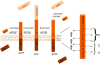
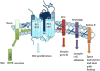
Similar articles
-
Complexity and Selectivity of γ-Secretase Cleavage on Multiple Substrates: Consequences in Alzheimer's Disease and Cancer.J Alzheimers Dis. 2018;61(1):1-15. doi: 10.3233/JAD-170628. J Alzheimers Dis. 2018. PMID: 29103038 Review.
-
Quantification of gamma-secretase modulation differentiates inhibitor compound selectivity between two substrates Notch and amyloid precursor protein.Mol Brain. 2008 Nov 4;1:15. doi: 10.1186/1756-6606-1-15. Mol Brain. 2008. PMID: 18983676 Free PMC article.
-
Amyloid-β protein (Aβ) Glu11 is the major β-secretase site of β-site amyloid-β precursor protein-cleaving enzyme 1(BACE1), and shifting the cleavage site to Aβ Asp1 contributes to Alzheimer pathogenesis.Eur J Neurosci. 2013 Jun;37(12):1962-9. doi: 10.1111/ejn.12235. Eur J Neurosci. 2013. PMID: 23773065
-
Gamma Secretase as an Important Drug Target for Management of Alzheimer's Disease: A Comprehensive Review.Curr Top Med Chem. 2024;24(2):109-127. doi: 10.2174/0115680266259174231006070637. Curr Top Med Chem. 2024. PMID: 37818580 Review.
-
BIIB042, a novel γ-secretase modulator, reduces amyloidogenic Aβ isoforms in primates and rodents and plaque pathology in a mouse model of Alzheimer's disease.Neuropharmacology. 2016 Apr;103:57-68. doi: 10.1016/j.neuropharm.2015.12.006. Epub 2015 Dec 12. Neuropharmacology. 2016. PMID: 26690893
Cited by
-
Expression of Amyloid Precursor Protein, Caveolin-1, Alpha-, Beta-, and Gamma-Secretases in Penumbra Cells after Photothrombotic Stroke and Evaluation of Neuroprotective Effect of Secretase and Caveolin-1 Inhibitors.Biomedicines. 2022 Oct 20;10(10):2655. doi: 10.3390/biomedicines10102655. Biomedicines. 2022. PMID: 36289917 Free PMC article.
-
Coupled Transmembrane Substrate Docking and Helical Unwinding in Intramembrane Proteolysis of Amyloid Precursor Protein.Sci Rep. 2018 Aug 17;8(1):12411. doi: 10.1038/s41598-018-30015-6. Sci Rep. 2018. PMID: 30120254 Free PMC article.
-
Medroxyprogesterone Acetate Impairs Amyloid Beta Degradation in a Matrix Metalloproteinase-9 Dependent Manner.Front Aging Neurosci. 2020 Apr 7;12:92. doi: 10.3389/fnagi.2020.00092. eCollection 2020. Front Aging Neurosci. 2020. PMID: 32317959 Free PMC article.
-
Amyloid beta: structure, biology and structure-based therapeutic development.Acta Pharmacol Sin. 2017 Sep;38(9):1205-1235. doi: 10.1038/aps.2017.28. Epub 2017 Jul 17. Acta Pharmacol Sin. 2017. PMID: 28713158 Free PMC article. Review.
-
Alzheimer's disease linked Aβ42 exerts product feedback inhibition on γ-secretase impairing downstream cell signaling.Elife. 2024 Jul 19;12:RP90690. doi: 10.7554/eLife.90690. Elife. 2024. PMID: 39027984 Free PMC article.
References
-
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. - PubMed
-
- Andersen OM, Reiche J, Schmidt V, Gotthardt M, Spoelgen R, Behlke J, von Arnim CA, Breiderhoff T, Jansen P, Wu X, Bales KR, Cappai R, Masters CL, Gliemann J, Mufson EJ, Hyman BT, Paul SM, Nykjaer A, Willnow TE. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc Natl Acad Sci U S A. 2005;102:13461–13466. - PMC - PubMed
-
- Annaert WG, Levesque L, Craessaerts K, Dierinck I, Snellings G, Westaway D, George-Hyslop PS, Cordell B, Fraser P, De Strooper B. Presenilin 1 controls gamma-secretase processing of amyloid precursor protein in pre-golgi compartments of hippocampal neurons. J Cell Biol. 1999;147:277–294. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

