Polarizing the Neuron through Sustained Co-expression of Alternatively Spliced Isoforms
- PMID: 27134173
- PMCID: PMC4870516
- DOI: 10.1016/j.celrep.2016.04.012
Polarizing the Neuron through Sustained Co-expression of Alternatively Spliced Isoforms
Abstract
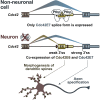
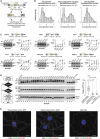
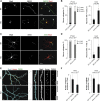
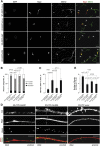
Alternative splicing (AS) is an important source of proteome diversity in eukaryotes. However, how this affects protein repertoires at a single-cell level remains an open question. Here, we show that many 3'-terminal exons are persistently co-expressed with their alternatives in mammalian neurons. In an important example of this scenario, cell polarity gene Cdc42, a combination of polypyrimidine tract-binding, protein-dependent, and constitutive splicing mechanisms ensures a halfway switch from the general (E7) to the neuron-specific (E6) alternative 3'-terminal exon during neuronal differentiation. Perturbing the nearly equimolar E6/E7 ratio in neurons results in defects in both axonal and dendritic compartments and suggests that Cdc42E7 is involved in axonogenesis, whereas Cdc42E6 is required for normal development of dendritic spines. Thus, co-expression of a precise blend of functionally distinct splice isoforms rather than a complete switch from one isoform to another underlies proper structural and functional polarization of neurons.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Ameur A., Zaghlool A., Halvardson J., Wetterbom A., Gyllensten U., Cavelier L., Feuk L. Total RNA sequencing reveals nascent transcription and widespread co-transcriptional splicing in the human brain. Nat. Struct. Mol. Biol. 2011;18:1435–1440. - PubMed
-
- Andreassi C., Riccio A. To localize or not to localize: mRNA fate is in 3’UTR ends. Trends Cell Biol. 2009;19:465–474. - PubMed
-
- Cappello S., Attardo A., Wu X., Iwasato T., Itohara S., Wilsch-Bräuninger M., Eilken H.M., Rieger M.A., Schroeder T.T., Huttner W.B. The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat. Neurosci. 2006;9:1099–1107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

