Anti-inflammatory activities of Ganoderma lucidum (Lingzhi) and San-Miao-San supplements in MRL/lpr mice for the treatment of systemic lupus erythematosus
- PMID: 27134645
- PMCID: PMC4851790
- DOI: 10.1186/s13020-016-0093-x
Anti-inflammatory activities of Ganoderma lucidum (Lingzhi) and San-Miao-San supplements in MRL/lpr mice for the treatment of systemic lupus erythematosus
Abstract
Background: Ganoderma lucidum (Lingzhi; LZ) and San-Miao-San (SMS) are Chinese medicines (CMs) used to treat inflammatory ailments and numbing syndrome/arthralgia syndrome (Bi Zheng), respectively. Given that the main symptoms of systemic lupus erythematosus (SLE) include inflammation of the joints, joint pain, edema and palpitations of the heart because of problems associated with Bi Zheng, it was envisaged that LZ and SMS could be used as potential treatments for this autoimmune disease. This study aims to investigate the anti-inflammatory activity of a combination formulation containing LZ and SMS (LZ-SMS) in SLE mice.
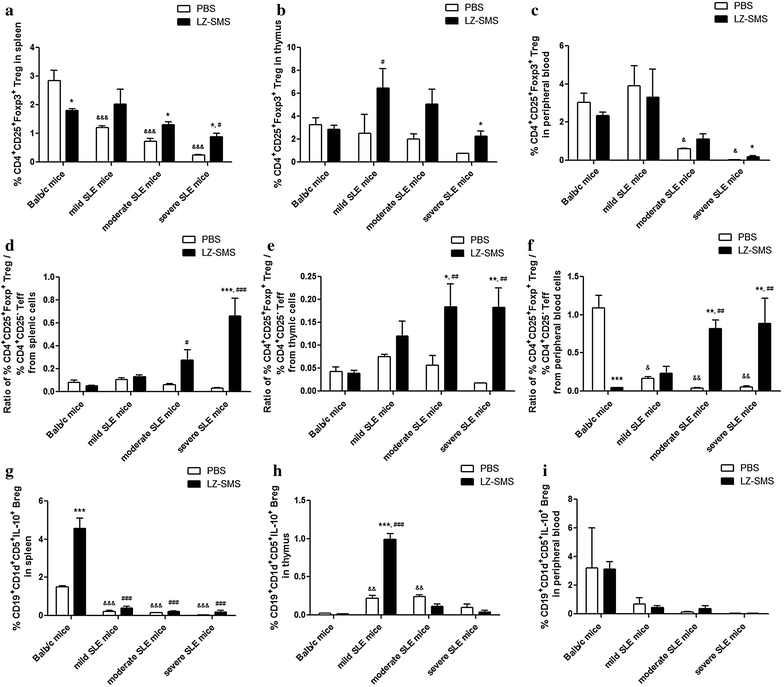
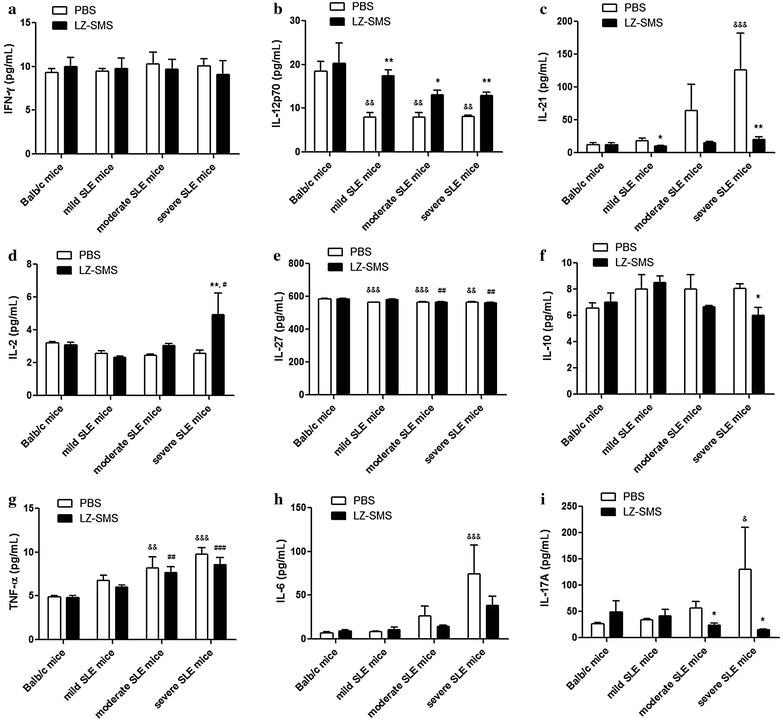
Methods: Female adult Balb/c mice of 20-24 weeks of age were used as normal mice (n = 10), whereas female MRL/lpr mice of 12-24 weeks of age were divided into three groups (n = 10 in each group), including mild, moderate and severe SLE mice groups. The clinical characteristics of the SLE and Babl/c mice (i.e., body weight, joint thickness, lupus flare, proteinuria, leukocyturia and lymphadenopathy) were assessed. The plasma concentrations of anti-nuclear antibody (ANA) and anti-double stranded DNA antibody (anti-ds-DNA) were analyzed by an enzyme-linked immunosorbent assay, whereas the concentration of several key cytokines (IFN-γ, TNF-α, IL-6, IL-10, IL-2, IL-27, IL-12P70, IL-17A and IL-21) were analyzed by a Luminex multiplex assay. The gene expression profiles for differentiation of the T helper (Th) lymphocytes in splenic CD4(+) Th cells were assessed by RT-qPCR. Flow cytometry was used to measure the percentages of CD4(+)CD25(+)Foxp3(+) Treg cells and CD19(+)CD5(+)CD1d(+)IL-10(+) regulatory B (Breg) cells (IL-10(+) Bregs).
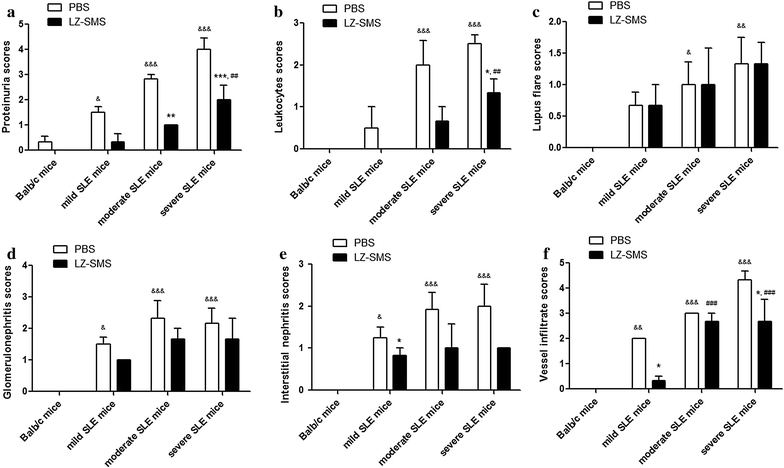
Results: Concentrations of anti-ds-DNA in the plasma samples collected from the LZ-SMS-treated (500 mg/kg/day oral administration for 7 days followed with 50 mg/kg/day intraperitoneal administration for 7 days), moderate and severe SLE mice decreased significantly compared with the PBS treated mice (P < 0.05). The gene expression levels of the induced regulatory T (iTreg) and natural Treg (nTreg) cells were significantly higher than those of the Th17, Th1 and "conventional Th cells vs. Treg cells" regulated genes following the LZ-SMS treatment (P < 0.05). The percentages of CD4(+)CD25(+)Foxp3(+) Treg cells collected from the splenic, thymic and peripheral blood cells, as well as the percentages of IL-10(+) Bregs collected from the splenic and thymic cells increased significantly in the LZ-SMS-treated SLE mice (P < 0.05) compared with the untreated PBS group. The ratio of the percentage of CD4(+)CD25(+)Foxp3(+) Treg cells to the percentage of CD4(+)CD25(-) effector T cells collected from the splenic, thymic and peripheral blood cells in LZ-SMS-treated moderate and severe SLE mice increased significantly compared with the untreated PBS group (P < 0.05). Furthermore, a comparison with the PBS treatment group revealed significant decreases in the concentrations of several inflammatory cytokines, including IL-21, IL-10 and IL-17A (P < 0.05), as well as significant increases in the concentrations of IL-2 and IL-12P70 in the LZ-SMS treated SLE mice (P < 0.05).
Conclusion: LZ-SMS treatment led to significant increases in the percentages of CD4(+)CD25(+)Foxp3(+) Treg and IL-10(+) Breg cells, together with a reduction in the plasma concentrations of several inflammatory cytokines and the down-regulated expression of the corresponding cytokine related genes in SLE mice. The clinical characteristics of the LZ-SMS-treated SLE mice also improved significantly.
Figures


Similar articles
-
Remission of systemic lupus erythematosus disease activity with regulatory cytokine interleukin (IL)-35 in Murphy Roths Large (MRL)/lpr mice.Clin Exp Immunol. 2015 Aug;181(2):253-66. doi: 10.1111/cei.12639. Clin Exp Immunol. 2015. PMID: 25845911 Free PMC article.
-
Aberrant expression of regulatory cytokine IL-35 in patients with systemic lupus erythematosus.Lupus. 2015 Oct;24(12):1257-66. doi: 10.1177/0961203315585815. Epub 2015 May 11. Lupus. 2015. PMID: 25966926
-
Role of CD19+ CD5+ CD1d+ Bregs in maintaining the Th17/Treg balance in mice with systemic lupus erythematosus complicated with atherosclerosis.Int J Rheum Dis. 2023 Jun;26(6):1048-1057. doi: 10.1111/1756-185X.14691. Epub 2023 Apr 3. Int J Rheum Dis. 2023. PMID: 37012219
-
IL-17A-Producing Foxp3+ Regulatory T Cells and Human Diseases.Immune Netw. 2017 Oct;17(5):276-286. doi: 10.4110/in.2017.17.5.276. Epub 2017 Oct 16. Immune Netw. 2017. PMID: 29093649 Free PMC article. Review.
-
Paradoxical role of Breg-inducing cytokines in autoimmune diseases.J Transl Autoimmun. 2019 Aug 17;2:100011. doi: 10.1016/j.jtauto.2019.100011. eCollection 2019 Dec. J Transl Autoimmun. 2019. PMID: 32743499 Free PMC article. Review.
Cited by
-
A Review of the Potential Benefits of Herbal Medicines, Small Molecules of Natural Sources, and Supplements for Health Promotion in Lupus Conditions.Life (Basel). 2023 Jul 19;13(7):1589. doi: 10.3390/life13071589. Life (Basel). 2023. PMID: 37511964 Free PMC article. Review.
-
In vitro evaluation of the synergistic antioxidant and anti-inflammatory activities of the combined extracts from Malaysian Ganoderma lucidum and Egyptian Chlorella vulgaris.BMC Complement Altern Med. 2018 May 10;18(1):154. doi: 10.1186/s12906-018-2218-5. BMC Complement Altern Med. 2018. PMID: 29747629 Free PMC article.
-
Lingzhi and San-Miao-San with hyaluronic acid gel mitigate cartilage degeneration in anterior cruciate ligament transection induced osteoarthritis.J Orthop Translat. 2020 Oct 21;26:132-140. doi: 10.1016/j.jot.2020.07.008. eCollection 2021 Jan. J Orthop Translat. 2020. PMID: 33437632 Free PMC article.
-
A Placebo-Controlled, Pseudo-Randomized, Crossover Trial of Botanical Agents for Gulf War Illness: Reishi Mushroom (Ganoderma lucidum), Stinging Nettle (Urtica dioica), and Epimedium (Epimedium sagittatum).Int J Environ Res Public Health. 2021 Apr 1;18(7):3671. doi: 10.3390/ijerph18073671. Int J Environ Res Public Health. 2021. PMID: 33915962 Free PMC article. Clinical Trial.
-
The Role of TLR7 and TLR9 in the Pathogenesis of Systemic Sclerosis.Int J Mol Sci. 2024 Jun 1;25(11):6133. doi: 10.3390/ijms25116133. Int J Mol Sci. 2024. PMID: 38892317 Free PMC article.
References
-
- Ippolito A, Petri M. An update on mortality in systemic lupus erythematosus. Clin Exp Rheumatol. 2008;26:S72–S79. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

