Certain Malvaceae Plants Have a Unique Accumulation of myo-Inositol 1,2,4,5,6-Pentakisphosphate
- PMID: 27135328
- PMCID: PMC4844327
- DOI: 10.3390/plants4020267
Certain Malvaceae Plants Have a Unique Accumulation of myo-Inositol 1,2,4,5,6-Pentakisphosphate
Abstract
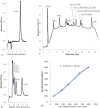
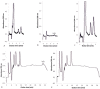
Methods used to quantify inositol phosphates in seeds lack the sensitivity and specificity necessary to accurately detect the lower concentrations of these compounds contained in the leaves of many plants. In order to measure inositol hexakisphosphate (InsP₆) and inositol pentakisphosphate (InsP₅) levels in leaves of different plants, a method was developed to concentrate and pre-purify these compounds prior to analysis. Inositol phosphates were extracted from leaves with diluted HCl and concentrated on small anion exchange columns. Reversed-phase solid phase extraction cartridges were used to remove compounds that give peaks that sometimes interfere during HPLC. The method permitted the determination of InsP₆ and InsP₅ concentrations in leaves as low as 10 µM and 2 µM, respectively. Most plants analyzed contained a high ratio of InsP₆ to InsP₅. In contrast, certain members of the Malvaceae family, such as cotton (Gossypium) and some hibiscus (Hibiscus) species, had a preponderance of InsP₅. Radiolabeling of cotton seedlings also showed increased amounts of InsP₅ relative to InsP₆. Why some Malvaceae species exhibit a reversal of the typical ratios of these inositol phosphates is an intriguing question for future research.
Keywords: Malvaceae; cotton; hexakisphosphate; inositol phosphates; pentakisphosphate; phosphate; phytate; phytic acid.
Figures



Similar articles
-
Regulation of inositol 1,2,4,5,6-pentakisphosphate and inositol hexakisphosphate levels in Gossypium hirsutum by IPK1.Planta. 2023 Jan 25;257(2):46. doi: 10.1007/s00425-023-04080-9. Planta. 2023. PMID: 36695941
-
myo-inositol pentakisphosphates. Structure, biological occurrence and phosphorylation to myo-inositol hexakisphosphate.Biochem J. 1991 Apr 15;275 ( Pt 2)(Pt 2):485-99. doi: 10.1042/bj2750485. Biochem J. 1991. PMID: 1850990 Free PMC article.
-
Phytic acid synthesis and vacuolar accumulation in suspension-cultured cells of Catharanthus roseus induced by high concentration of inorganic phosphate and cations.Plant Physiol. 2005 Jul;138(3):1607-14. doi: 10.1104/pp.105.060269. Epub 2005 Jun 17. Plant Physiol. 2005. PMID: 15965017 Free PMC article.
-
Assessing the omnipotence of inositol hexakisphosphate.Cell Signal. 2001 Mar;13(3):151-8. doi: 10.1016/s0898-6568(01)00129-2. Cell Signal. 2001. PMID: 11282453 Review.
-
Biosynthesis and possible functions of inositol pyrophosphates in plants.Front Plant Sci. 2015 Feb 12;6:67. doi: 10.3389/fpls.2015.00067. eCollection 2015. Front Plant Sci. 2015. PMID: 25729385 Free PMC article. Review.
Cited by
-
The inositol pyrophosphate synthesis pathway in Trypanosoma brucei is linked to polyphosphate synthesis in acidocalcisomes.Mol Microbiol. 2017 Oct;106(2):319-333. doi: 10.1111/mmi.13766. Epub 2017 Aug 22. Mol Microbiol. 2017. PMID: 28792096 Free PMC article.
-
Intracellular phosphate sensing and regulation of phosphate transport systems in plants.Plant Physiol. 2021 Dec 4;187(4):2043-2055. doi: 10.1093/plphys/kiab343. Plant Physiol. 2021. PMID: 35235674 Free PMC article. Review.
References
-
- Stevenson-Paulik J., Phillippy B.Q. Inositol polyphosphates and kinases. In: Minnik T., editor. Lipid Signaling in Plants. Volume 16. Springer-Verlag Berlin; Berlin, Germany: 2010. pp. 161–174. (Book Series: Plant Cell Monographs).
LinkOut - more resources
Full Text Sources
Other Literature Sources

