Slow Replication Fork Velocity of Homologous Recombination-Defective Cells Results from Endogenous Oxidative Stress
- PMID: 27135742
- PMCID: PMC4852921
- DOI: 10.1371/journal.pgen.1006007
Slow Replication Fork Velocity of Homologous Recombination-Defective Cells Results from Endogenous Oxidative Stress
Abstract
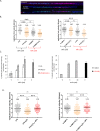
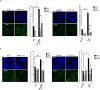
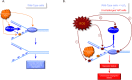
Replications forks are routinely hindered by different endogenous stresses. Because homologous recombination plays a pivotal role in the reactivation of arrested replication forks, defects in homologous recombination reveal the initial endogenous stress(es). Homologous recombination-defective cells consistently exhibit a spontaneously reduced replication speed, leading to mitotic extra centrosomes. Here, we identify oxidative stress as a major endogenous source of replication speed deceleration in homologous recombination-defective cells. The treatment of homologous recombination-defective cells with the antioxidant N-acetyl-cysteine or the maintenance of the cells at low O2 levels (3%) rescues both the replication fork speed, as monitored by single-molecule analysis (molecular combing), and the associated mitotic extra centrosome frequency. Reciprocally, the exposure of wild-type cells to H2O2 reduces the replication fork speed and generates mitotic extra centrosomes. Supplying deoxynucleotide precursors to H2O2-exposed cells rescued the replication speed. Remarkably, treatment with N-acetyl-cysteine strongly expanded the nucleotide pool, accounting for the replication speed rescue. Remarkably, homologous recombination-defective cells exhibit a high level of endogenous reactive oxygen species. Consistently, homologous recombination-defective cells accumulate spontaneous γH2AX or XRCC1 foci that are abolished by treatment with N-acetyl-cysteine or maintenance at 3% O2. Finally, oxidative stress stimulated homologous recombination, which is suppressed by supplying deoxynucleotide precursors. Therefore, the cellular redox status strongly impacts genome duplication and transmission. Oxidative stress should generate replication stress through different mechanisms, including DNA damage and nucleotide pool imbalance. These data highlight the intricacy of endogenous replication and oxidative stresses, which are both evoked during tumorigenesis and senescence initiation, and emphasize the importance of homologous recombination as a barrier against spontaneous genetic instability triggered by the endogenous oxidative/replication stress axis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005. pp. 864–870. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

