A Placebo-Controlled Study on the Effects of the Glucagon-Like Peptide-1 Mimetic, Exenatide, on Insulin Secretion, Body Composition and Adipokines in Obese, Client-Owned Cats
- PMID: 27136422
- PMCID: PMC4852899
- DOI: 10.1371/journal.pone.0154727
A Placebo-Controlled Study on the Effects of the Glucagon-Like Peptide-1 Mimetic, Exenatide, on Insulin Secretion, Body Composition and Adipokines in Obese, Client-Owned Cats
Abstract
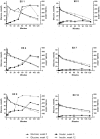
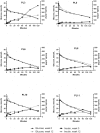
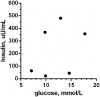
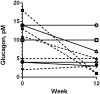
Glucagon-like Peptide-1 mimetics increase insulin secretion and reduces body weight in humans. In lean, healthy cats, short-term treatment has produced similar results, whereas the effect in obese cats or with extended duration of treatment is unknown. Here, prolonged (12 weeks) treatment with the Glucagon-like Peptide-1 mimetic, exenatide, was evaluated in 12 obese, but otherwise healthy, client-owned cats. Cats were randomized to exenatide (1.0 μg/kg) or placebo treatment twice daily for 12 weeks. The primary endpoint was changes in insulin concentration; the secondary endpoints were glucose homeostasis, body weight, body composition as measured by dual-energy x-ray absorptiometry and overall safety. An intravenous glucose tolerance test (1 g/kg body weight) was conducted at week 0 and week 12. Exenatide did not change the insulin concentration, plasma glucose concentration or glucose tolerance (P>0.05 for all). Exenatide tended to reduce body weight on continued normal feeding. Median relative weight loss after 12 weeks was 5.1% (range 1.7 to 8.4%) in the exenatide group versus 3.2% (range -5.3 to 5.7%) in the placebo group (P = 0.10). Body composition and adipokine levels were unaffected by exenatide (P>0.05). Twelve weeks of exenatide was well-tolerated, with only two cases of mild, self-limiting gastrointestinal signs and a single case of mild hypoglycemia. The long-term insulinotropic effect of exenatide appeared less pronounced in obese cats compared to previous short-term studies in lean cats. Further investigations are required to fully elucidate the effect on insulin secretion, glucose tolerance and body weight in obese cats.
Conflict of interest statement
Figures

Similar articles
-
The GLP-1 mimetic exenatide potentiates insulin secretion in healthy cats.Domest Anim Endocrinol. 2011 Jul;41(1):42-9. doi: 10.1016/j.domaniend.2011.03.001. Epub 2011 Apr 6. Domest Anim Endocrinol. 2011. PMID: 21645806 Clinical Trial.
-
Pharmacology of the glucagon-like peptide-1 analog exenatide extended-release in healthy cats.Domest Anim Endocrinol. 2015 Apr;51:78-85. doi: 10.1016/j.domaniend.2014.12.003. Epub 2014 Dec 20. Domest Anim Endocrinol. 2015. PMID: 25594949
-
Effect of the Glucagon-like Peptide-1 Analogue Exenatide Extended Release in Cats with Newly Diagnosed Diabetes Mellitus.J Vet Intern Med. 2016 Jan-Feb;30(1):92-100. doi: 10.1111/jvim.13817. Epub 2015 Dec 24. J Vet Intern Med. 2016. PMID: 26700409 Free PMC article. Clinical Trial.
-
Exenatide and weight loss.Nutrition. 2010 Mar;26(3):243-9. doi: 10.1016/j.nut.2009.07.008. Nutrition. 2010. PMID: 20152707 Review.
-
Clinical efficacy and safety of once-weekly glucagon-like peptide-1 agonists in development for treatment of type 2 diabetes mellitus in adults.Ann Pharmacother. 2012 Jan;46(1):68-78. doi: 10.1345/aph.1Q379. Ann Pharmacother. 2012. PMID: 22232377 Review.
Cited by
-
Safety, Tolerability, and Proof-Of-Concept Study of OKV-119, a Novel Exenatide Long-Term Drug Delivery System, in Healthy Cats.Front Vet Sci. 2021 May 11;8:661546. doi: 10.3389/fvets.2021.661546. eCollection 2021. Front Vet Sci. 2021. PMID: 34046446 Free PMC article.
-
Physiological and pharmacological actions of glucagon like peptide-1 (GLP-1) in domestic animals.Vet Anim Sci. 2022 Mar 23;16:100245. doi: 10.1016/j.vas.2022.100245. eCollection 2022 Jun. Vet Anim Sci. 2022. PMID: 35372707 Free PMC article.
References
-
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 2007;87(4):1409–39. - PubMed
-
- Goke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J, et al. Exendin-4 is a high potency agonist and truncated exendin-(9–39)-amide an antagonist at the glucagon-like peptide 1-(7–36)-amide receptor of insulin-secreting beta-cells. J Biol Chem 1993;268(26):19650–5. - PubMed
-
- Kolterman OG, Kim DD, Shen L, Ruggles JA, Nielsen LL, Fineman MS, et al. Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health System Pharm 2005;62(2):173–81. - PubMed
-
- Moretto TJ, Milton DR, Ridge TD, Macconell LA, Okerson T, Wolka AM, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2008;30(8):1448–60. 10.1016/j.clinthera.2008.08.006 - DOI - PubMed
-
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006;368(9548):1696–705. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

