Synaptic pruning in the female hippocampus is triggered at puberty by extrasynaptic GABAA receptors on dendritic spines
- PMID: 27136678
- PMCID: PMC4871702
- DOI: 10.7554/eLife.15106
Synaptic pruning in the female hippocampus is triggered at puberty by extrasynaptic GABAA receptors on dendritic spines
Abstract
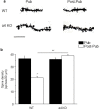
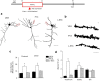
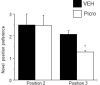
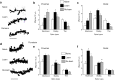
Adolescent synaptic pruning is thought to enable optimal cognition because it is disrupted in certain neuropathologies, yet the initiator of this process is unknown. One factor not yet considered is the α4βδ GABAA receptor (GABAR), an extrasynaptic inhibitory receptor which first emerges on dendritic spines at puberty in female mice. Here we show that α4βδ GABARs trigger adolescent pruning. Spine density of CA1 hippocampal pyramidal cells decreased by half post-pubertally in female wild-type but not α4 KO mice. This effect was associated with decreased expression of kalirin-7 (Kal7), a spine protein which controls actin cytoskeleton remodeling. Kal7 decreased at puberty as a result of reduced NMDAR activation due to α4βδ-mediated inhibition. In the absence of this inhibition, Kal7 expression was unchanged at puberty. In the unpruned condition, spatial re-learning was impaired. These data suggest that pubertal pruning requires α4βδ GABARs. In their absence, pruning is prevented and cognition is not optimal.
Keywords: GABA-A receptor; cell biology; delta; dendritic spine; hippocampus; mouse; neuroscience; puberty; synaptic pruning.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures








References
-
- Aoki C, Sabaliauskas N, Chowdhury T, Min JY, Colacino AR, Laurino K, Barbarich-Marsteller NC. Adolescent female rats exhibiting activity-based anorexia express elevated levels of GABA(A) receptor α4 and δ subunits at the plasma membrane of hippocampal CA1 spines. Synapse. 2012;66:391–407. doi: 10.1002/syn.21528. - DOI - PMC - PubMed
-
- Bannerman DM, Niewoehner B, Lyon L, Romberg C, Schmitt WB, Taylor A, Sanderson DJ, Cottam J, Sprengel R, Seeburg PH, Köhr G, Rawlins JN, Köhr G, Rawlins JN. NMDA receptor subunit NR2A is required for rapidly acquired spatial working memory but not incremental spatial reference memory. Journal of Neuroscience. 2008;28:3623–3630. doi: 10.1523/JNEUROSCI.3639-07.2008. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

