Neuropsychiatric Disturbances in Alzheimer's Disease: What Have We Learned from Neuropathological Studies?
- PMID: 27137218
- PMCID: PMC5070416
- DOI: 10.2174/1567205013666160502123607
Neuropsychiatric Disturbances in Alzheimer's Disease: What Have We Learned from Neuropathological Studies?
Abstract
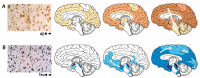
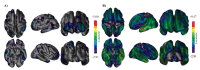
Neuropsychiatric symptoms (NPS) are an integral part of the dementia syndrome and were therefore recently included in the core diagnostic criteria of dementia. The near universal prevalence of NPS in Alzheimer's disease (AD), combined with their disabling effects on patients and caregivers, is contrasted by the fact that few effective and safe treatments exist, which is in part to be attributed to our incomplete understanding of the neurobiology of NPS. In this review, we describe the pathological alterations typical for AD, including spreading and evolution of burden, effect on the molecular and cellular integrity, functional consequences and atrophy of NPS-relevant brain regions and circuits in correlation with specific NPS assessments. It is thereby clearly established that NPS are fundamental expressions of the underlying neurodegenerative brain disease and not simply reflect the patients' secondary response to their illness. Neuropathological studies, moreover, include a majority of end-stage patient samples, which may not correctly represent the pathophysiological environment responsible for particular NPS that may already be present in an early stage, or even prior to AD diagnosis. The burdensome nature and high prevalence of NPS, in combination with the absence of effective and safe pharmacotherapies, provide a strong incentive to continue neuropathological and neurochemical, as well as imaging and other relevant approaches to further improve our apprehension of the neurobiology of NPS.
Figures


Similar articles
-
Neuropsychiatric Symptoms in Alzheimer Disease, Vascular Dementia, and Mixed Dementia.Neurodegener Dis. 2017;17(4-5):127-134. doi: 10.1159/000455127. Epub 2017 Mar 1. Neurodegener Dis. 2017. PMID: 28245482
-
Impact of cerebrovascular pathology on behavioural and neuropsychiatric symptoms in patients with Alzheimer's dementia: findings from a retrospective, naturalistic study.Int J Clin Pract. 2009 Jul;63(7):1024-30. doi: 10.1111/j.1742-1241.2009.02079.x. Int J Clin Pract. 2009. PMID: 19570120
-
Prevalence of neuropsychiatric symptoms in mild cognitive impairment and Alzheimer's disease, and its relationship with cognitive impairment.Curr Alzheimer Res. 2010 Sep;7(6):517-26. doi: 10.2174/156720510792231748. Curr Alzheimer Res. 2010. PMID: 20455862
-
Alzheimer's disease and its focal variants.Semin Neurol. 2000;20(4):447-54. doi: 10.1055/s-2000-13177. Semin Neurol. 2000. PMID: 11149700 Review.
-
Apolipoprotein E genotypes and neuropsychiatric symptoms and syndromes in late-onset Alzheimer's disease.Ageing Res Rev. 2012 Jan;11(1):87-103. doi: 10.1016/j.arr.2011.06.005. Epub 2011 Jul 7. Ageing Res Rev. 2012. PMID: 21763789 Review.
Cited by
-
Neuroanatomical associations of depression, anxiety and apathy neuropsychiatric symptoms in patients with Alzheimer's disease.Acta Neurol Belg. 2021 Dec;121(6):1469-1480. doi: 10.1007/s13760-020-01349-8. Epub 2020 Apr 21. Acta Neurol Belg. 2021. PMID: 32319015
-
Acupuncture for Behavioral and Psychological Symptoms of Dementia: A Systematic Review and Meta-Analysis.J Clin Med. 2021 Jul 13;10(14):3087. doi: 10.3390/jcm10143087. J Clin Med. 2021. PMID: 34300254 Free PMC article. Review.
-
MicroRNA-138 Overexpression Alters Aβ42 Levels and Behavior in Wildtype Mice.Front Neurosci. 2021 Jan 14;14:591138. doi: 10.3389/fnins.2020.591138. eCollection 2020. Front Neurosci. 2021. PMID: 33519353 Free PMC article.
-
Cerebral Volumetric Correlates of Apathy in Alzheimer's Disease and Cognitively Normal Older Adults: Meta-Analysis, Label-Based Review, and Study of an Independent Cohort.J Alzheimers Dis. 2022;85(3):1251-1265. doi: 10.3233/JAD-215316. J Alzheimers Dis. 2022. PMID: 34924392 Free PMC article.
-
Searching for new drugs for the treatment of dementia-related psychosis.Postep Psychiatr Neurol. 2021 Dec;30(4):270-277. doi: 10.5114/ppn.2021.111942. Epub 2021 Dec 21. Postep Psychiatr Neurol. 2021. PMID: 37082556 Free PMC article. Review.
References
-
- Reisberg B., Borenstein J., Salob S.P., Ferris S.H., Franssen E., Georgotas A. Behavioral symptoms in Alzheimer’s disease: Phenomenology and treatment. J. Clin. Psychiatry. 1987;48:9–15. - PubMed
-
- Cummings J.L., Mega M., Gray K., Rosenberg-Thompson S., Carusi D.A., Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. - PubMed
-
- Mega M.S., Cummings J.L., Fiorello T., Gornbein J. The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 1996;46:130–135. - PubMed

