The role of long-term label-retaining cells in the regeneration of adult mouse kidney after ischemia/reperfusion injury
- PMID: 27137761
- PMCID: PMC4852428
- DOI: 10.1186/s13287-016-0324-1
The role of long-term label-retaining cells in the regeneration of adult mouse kidney after ischemia/reperfusion injury
Abstract
Background: Label-retaining cells (LRCs) have been recognized as rare stem and progenitor-like cells, but their complex biological features in renal repair at the cellular level have never been reported. This study was conducted to evaluate whether LRCs in kidney are indeed renal stem/progenitor cells and to delineate their potential role in kidney regeneration.
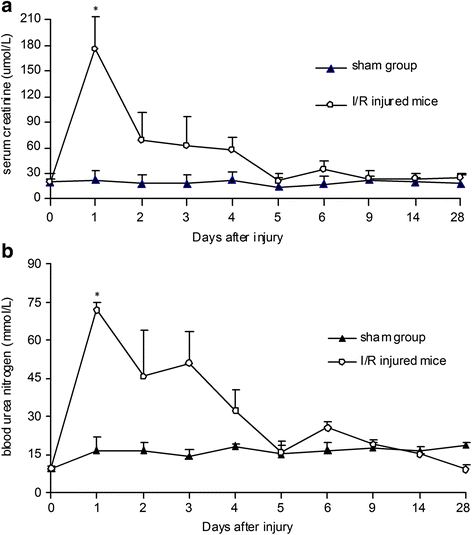
Methods: We utilized a long-term pulse chase of 5-bromo-2'-deoxyuridine (BrdU)-labeled cells in C57BL/6J mice to identify renal LRCs. We tracked the precise morphological characteristics and locations of BrdU(+)LRCs by both immunohistochemistry and immunofluorescence. To examine whether these BrdU(+)LRCs contribute to the repair of acute kidney injury, we analyzed biological characteristics of BrdU(+)LRCs in mice after ischemia/reperfusion (I/R) injury.
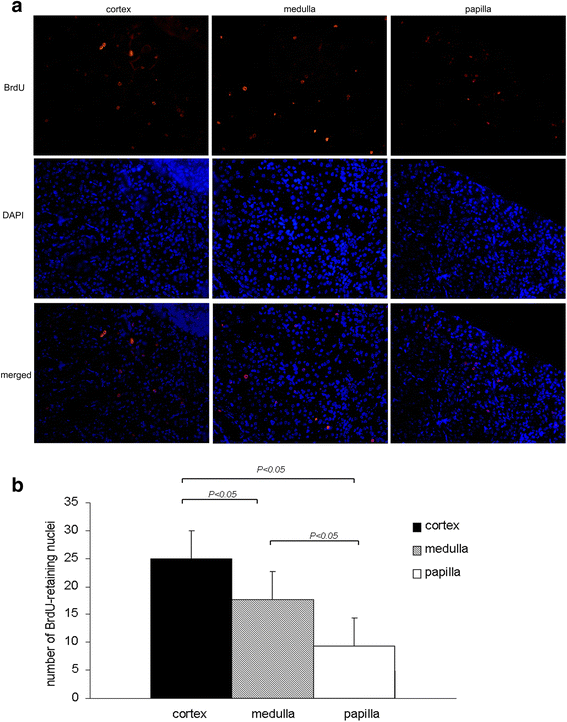
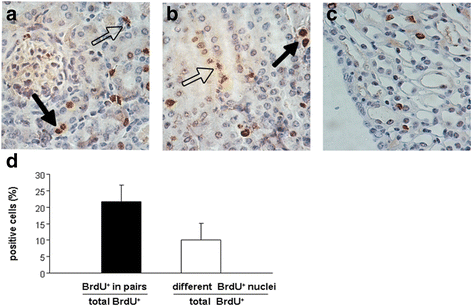
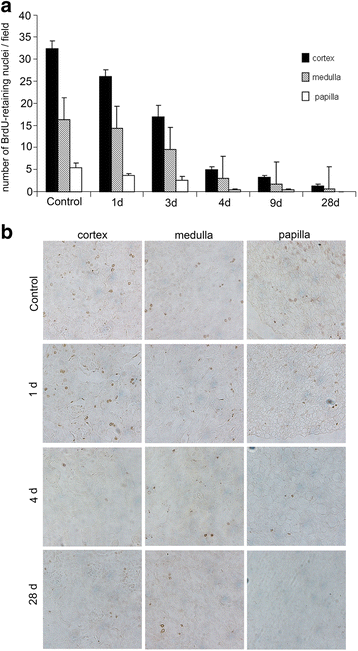
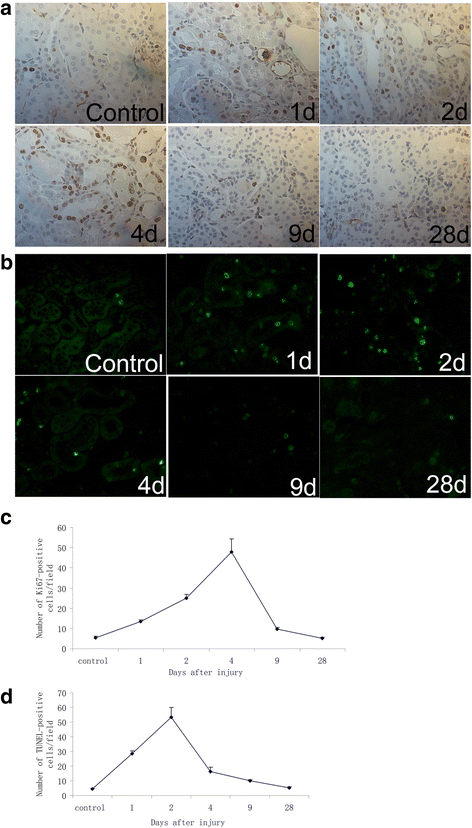
Results: The findings revealed that the nuclei of BrdU(+) LRCs exhibited different morphological characteristics in normal adult kidneys, including nuclei in pairs or scattered, fragmented or intact, strongly or weakly positive. Only 24.3 ± 1.5 % of BrdU(+) LRCs co-expressed with Ki67 and 9.1 ± 1.4 % of BrdU(+) LRCs were positive for TUNEL following renal I/R injury. Interestingly, we found that newly regenerated cells formed a niche-like structure and LRCs in pairs tended to locate in this structure, but the number of those LRCs was very low. We found a few scattered LRCs co-expressed Lotus tetragonolobus agglutinin (LTA) in the early phase of injury, suggesting differentiation of those LRCs in mouse kidney.
Conclusions: Our findings suggest that LRCs are not a simple type of slow-cycling cells in adult kidneys, indicating a limited role of these cells in the regeneration of I/R injured kidney. Thus, LRCs cannot reliably be considered stem/progenitor cells in the regeneration of adult mouse kidney. When researchers use this technique to study the cellular basis of renal repair, these complex features of renal LRCs and the purity of real stem cells among renal LRCs should be considered.
Keywords: AKI; Ischemia/reperfusion injury; Kidney regeneration; Label-retaining cells; Renal progenitor cells; Renal stem cells.
Figures




Similar articles
-
Kinetics of Label Retaining Cells in the Developing Rat Kidneys.PLoS One. 2015 Dec 9;10(12):e0144734. doi: 10.1371/journal.pone.0144734. eCollection 2015. PLoS One. 2015. PMID: 26650841 Free PMC article.
-
Identification of label-retaining cells in mouse endometrium.Stem Cells. 2006 Jun;24(6):1529-38. doi: 10.1634/stemcells.2005-0411. Epub 2006 Feb 2. Stem Cells. 2006. PMID: 16456137
-
Age-related decline in label-retaining tubular cells: implication for reduced regenerative capacity after injury in the aging kidney.Am J Physiol Renal Physiol. 2012 Mar 15;302(6):F694-702. doi: 10.1152/ajprenal.00249.2011. Epub 2011 Dec 14. Am J Physiol Renal Physiol. 2012. PMID: 22169012
-
Injury-associated reacquiring of intestinal stem cell function.World J Gastroenterol. 2015 Feb 21;21(7):2005-10. doi: 10.3748/wjg.v21.i7.2005. World J Gastroenterol. 2015. PMID: 25717233 Free PMC article. Review.
-
Label-retaining cells in the kidney: origin of regenerating cells after renal ischemia.Clin Exp Nephrol. 2007 Dec;11(4):269-274. doi: 10.1007/s10157-007-0500-9. Epub 2007 Dec 21. Clin Exp Nephrol. 2007. PMID: 18085386 Review.
Cited by
-
Possible Underlying Mechanisms for the Renoprotective Effect of Retinoic Acid-Pretreated Wharton's Jelly Mesenchymal Stem Cells against Renal Ischemia/Reperfusion Injury.Cells. 2022 Jun 22;11(13):1997. doi: 10.3390/cells11131997. Cells. 2022. PMID: 35805083 Free PMC article.
-
Gcm1 is involved in cell proliferation and fibrosis during kidney regeneration after ischemia-reperfusion injury.Sci Rep. 2019 May 27;9(1):7883. doi: 10.1038/s41598-019-44161-y. Sci Rep. 2019. PMID: 31133638 Free PMC article.
-
Kidney Cells Regeneration: Dedifferentiation of Tubular Epithelium, Resident Stem Cells and Possible Niches for Renal Progenitors.Int J Mol Sci. 2019 Dec 15;20(24):6326. doi: 10.3390/ijms20246326. Int J Mol Sci. 2019. PMID: 31847447 Free PMC article. Review.
-
The Role of Long Term Label-Retaining Cells in the Treatment of Erectile Dysfunction by Vacuum Erectile Device.Sex Med. 2021 Dec;9(6):100442. doi: 10.1016/j.esxm.2021.100442. Epub 2021 Oct 11. Sex Med. 2021. PMID: 34649131 Free PMC article.
-
Phenotypic Analysis of BrdU Label-Retaining Cells during the Maturation of Conducting Airway Epithelium in a Porcine Lung.Stem Cells Int. 2019 Feb 27;2019:7043890. doi: 10.1155/2019/7043890. eCollection 2019. Stem Cells Int. 2019. PMID: 30936924 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

