Total Body Metabolic Tumor Response in ALK Positive Non-Small Cell Lung Cancer Patients Treated with ALK Inhibition
- PMID: 27137772
- PMCID: PMC4854442
- DOI: 10.1371/journal.pone.0149955
Total Body Metabolic Tumor Response in ALK Positive Non-Small Cell Lung Cancer Patients Treated with ALK Inhibition
Abstract
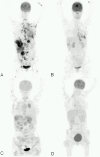
Background: In ALK-positive advanced NSCLC, crizotinib has a high response rate and effectively increases quality of life and survival. CT measurement of the tumor may insufficiently reflect the actual tumor load changes during targeted therapy with crizotinib. We explored whether 18F-FDG PET measured metabolic changes are different from CT based changes and studied the impact of these changes on disease progression.
Methods: 18F-FDG PET/CT was performed prior to and after 6 weeks of crizotinib treatment. Tumor response on CT was classified with RECIST 1.1, while 18F-FDG PET response was assessed according to the 1999 EORTC recommendations and PERCIST criteria. Agreement was assessed using McNemars test. During follow-up, patients received additional PET/CT during crizotinib treatment and second generation ALK inhibition. We assessed whether PET was able to detect progression earlier then CT.
Results: In this exploratory study 15 patients were analyzed who were treated with crizotinib. There was a good agreement in the applicability of CT and 18F-FDG PET/CT using the EORTC recommendations. During first line crizotinib and subsequent second line ALK inhibitors, PET was able to detect progression earlier then CT in 10/22 (45%) events of progression and in the others disease progression was detected simultaneously.
Conclusion: In advanced ALK positive NSCLC PET was able to detect progressive disease earlier than with CT in nearly half of the assessments while both imaging tests performed similar in the others.
Conflict of interest statement
Figures

References
-
- Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35(13):1773–82. Epub 2000/02/16. doi: S0959804999002294 [pii]. . - PubMed
-
- Sunaga N, Oriuchi N, Kaira K, Yanagitani N, Tomizawa Y, Hisada T, et al. Usefulness of FDG-PET for early prediction of the response to gefitinib in non-small cell lung cancer. Lung Cancer. 2008;59(2):203–10. Epub 2007/10/05. doi: S0169-5002(07)00482-5 [pii] 10.1016/j.lungcan.2007.08.012 . - DOI - PubMed
-
- Kobe C, Scheffler M, Holstein A, Zander T, Nogova L, Lammertsma AA, et al. Predictive value of early and late residual 18F-fluorodeoxyglucose and 18F-fluorothymidine uptake using different SUV measurements in patients with non-small-cell lung cancer treated with erlotinib. Eur J Nucl Med Mol Imaging. 2012;39(7):1117–27. Epub 2012/04/25. 10.1007/s00259-012-2118-8 . - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

