Functional and Structural Mimicry of Cellular Protein Kinase A Anchoring Proteins by a Viral Oncoprotein
- PMID: 27137912
- PMCID: PMC4854477
- DOI: 10.1371/journal.ppat.1005621
Functional and Structural Mimicry of Cellular Protein Kinase A Anchoring Proteins by a Viral Oncoprotein
Abstract
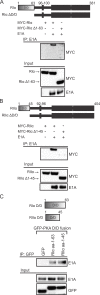
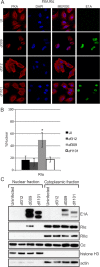
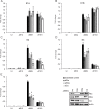
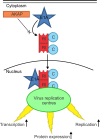
The oncoproteins of the small DNA tumor viruses interact with a plethora of cellular regulators to commandeer control of the infected cell. During infection, adenovirus E1A deregulates cAMP signalling and repurposes it for activation of viral gene expression. We show that E1A structurally and functionally mimics a cellular A-kinase anchoring protein (AKAP). E1A interacts with and relocalizes protein kinase A (PKA) to the nucleus, likely to virus replication centres, via an interaction with the regulatory subunits of PKA. Binding to PKA requires the N-terminus of E1A, which bears striking similarity to the amphipathic α-helical domain present in cellular AKAPs. E1A also targets the same docking-dimerization domain of PKA normally bound by cellular AKAPs. In addition, the AKAP like motif within E1A could restore PKA interaction to a cellular AKAP in which its normal interaction motif was deleted. During infection, E1A successfully competes with endogenous cellular AKAPs for PKA interaction. E1A's role as a viral AKAP contributes to viral transcription, protein expression and progeny production. These data establish HAdV E1A as the first known viral AKAP. This represents a unique example of viral subversion of a crucial cellular regulatory pathway via structural mimicry of the PKA interaction domain of cellular AKAPs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

