The Human Sodium-Glucose Cotransporter (hSGLT1) Is a Disulfide-Bridged Homodimer with a Re-Entrant C-Terminal Loop
- PMID: 27137918
- PMCID: PMC4854415
- DOI: 10.1371/journal.pone.0154589
The Human Sodium-Glucose Cotransporter (hSGLT1) Is a Disulfide-Bridged Homodimer with a Re-Entrant C-Terminal Loop
Abstract
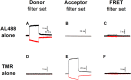
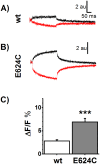
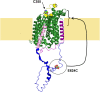
Na-coupled cotransporters are proteins that use the trans-membrane electrochemical gradient of Na to activate the transport of a second solute. The sodium-glucose cotransporter 1 (SGLT1) constitutes a well-studied prototype of this transport mechanism but essential molecular characteristics, namely its quaternary structure and the exact arrangement of the C-terminal transmembrane segments, are still debated. After expression in Xenopus oocytes, human SGLT1 molecules (hSGLT1) were labelled on an externally accessible cysteine residue with a thiol-reactive fluorophore (tetramethylrhodamine-C5-maleimide, TMR). Addition of dipicrylamine (DPA, a negatively-charged amphiphatic fluorescence "quencher") to the fluorescently-labelled oocytes is used to quench the fluorescence originating from hSGLT1 in a voltage-dependent manner. Using this arrangement with a cysteine residue introduced at position 624 in the loop between transmembrane segments 12 and 13, the voltage-dependent fluorescence signal clearly indicated that this portion of the 12-13 loop is located on the external side of the membrane. As the 12-13 loop begins on the intracellular side of the membrane, this suggests that the 12-13 loop is re-entrant. Using fluorescence resonance energy transfer (FRET), we observed that different hSGLT1 molecules are within molecular distances from each other suggesting a multimeric complex arrangement. In agreement with this conclusion, a western blot analysis showed that hSGLT1 migrates as either a monomer or a dimer in reducing and non-reducing conditions, respectively. A systematic mutational study of endogenous cysteine residues in hSGLT1 showed that a disulfide bridge is formed between the C355 residues of two neighbouring hSGLT1 molecules. It is concluded that, 1) hSGLT1 is expressed as a disulfide bridged homodimer via C355 and that 2) a portion of the intracellular 12-13 loop is re-entrant and readily accessible from the extracellular milieu.
Conflict of interest statement
Figures



Similar articles
-
Active site voltage clamp fluorometry of the sodium glucose cotransporter hSGLT1.Proc Natl Acad Sci U S A. 2017 Nov 14;114(46):E9980-E9988. doi: 10.1073/pnas.1713899114. Epub 2017 Oct 30. Proc Natl Acad Sci U S A. 2017. PMID: 29087341 Free PMC article.
-
Identification of a disulfide bridge linking the fourth and the seventh extracellular loops of the Na+/glucose cotransporter.J Gen Physiol. 2006 Feb;127(2):145-58. doi: 10.1085/jgp.200509439. J Gen Physiol. 2006. PMID: 16446504 Free PMC article.
-
High-yield functional expression of human sodium/d-glucose cotransporter1 in Pichia pastoris and characterization of ligand-induced conformational changes as studied by tryptophan fluorescence.Biochemistry. 2005 Nov 29;44(47):15514-24. doi: 10.1021/bi051377q. Biochemistry. 2005. PMID: 16300400
-
Conformational dynamics of hSGLT1 during Na+/glucose cotransport.J Gen Physiol. 2006 Dec;128(6):701-20. doi: 10.1085/jgp.200609643. J Gen Physiol. 2006. PMID: 17130520 Free PMC article.
-
Structure and function of the Na+/glucose cotransporter.Acta Physiol Scand Suppl. 1998 Aug;643:257-64. Acta Physiol Scand Suppl. 1998. PMID: 9789568 Review.
Cited by
-
Intestinal Saturated Long-Chain Fatty Acid, Glucose and Fructose Transporters and Their Inhibition by Natural Plant Extracts in Caco-2 Cells.Molecules. 2018 Oct 6;23(10):2544. doi: 10.3390/molecules23102544. Molecules. 2018. PMID: 30301205 Free PMC article. Review.
-
Diet management in congenital diarrheas and enteropathies - general concepts and disease-specific approach, a narrative review.Am J Clin Nutr. 2024 Jul;120(1):17-33. doi: 10.1016/j.ajcnut.2024.05.004. Epub 2024 May 9. Am J Clin Nutr. 2024. PMID: 38734141 Free PMC article. Review.
-
A comprehensive overview of substrate specificity of glycoside hydrolases and transporters in the small intestine : "A gut feeling".Cell Mol Life Sci. 2020 Dec;77(23):4799-4826. doi: 10.1007/s00018-020-03564-1. Epub 2020 Jun 6. Cell Mol Life Sci. 2020. PMID: 32506169 Free PMC article. Review.
-
Validation of sodium/glucose cotransporter proteins in human brain as a potential marker for temporal narrowing of the trauma formation.Int J Legal Med. 2019 Jul;133(4):1107-1114. doi: 10.1007/s00414-018-1893-6. Epub 2018 Aug 2. Int J Legal Med. 2019. PMID: 30073510
References
-
- Forrest LR, Krämer R, Ziegler C. The structural basis of secondary active transport mechanisms. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 2010. - PubMed
-
- Gagnon DG, Holt A, Bourgeois F, Wallendorff B, Coady MJ, Lapointe JY. Membrane topology of loop 13–14 of the Na+/glucose cotransporter (SGLT1): A SCAM and fluorescent labelling study. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2005;1712(2):173–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

