Design of a Genetically Stable High Fidelity Coxsackievirus B3 Polymerase That Attenuates Virus Growth in Vivo
- PMID: 27137934
- PMCID: PMC4933160
- DOI: 10.1074/jbc.M116.726596
Design of a Genetically Stable High Fidelity Coxsackievirus B3 Polymerase That Attenuates Virus Growth in Vivo
Abstract
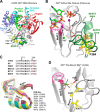
Positive strand RNA viruses replicate via a virally encoded RNA-dependent RNA polymerase (RdRP) that uses a unique palm domain active site closure mechanism to establish the canonical two-metal geometry needed for catalysis. This mechanism allows these viruses to evolutionarily fine-tune their replication fidelity to create an appropriate distribution of genetic variants known as a quasispecies. Prior work has shown that mutations in conserved motif A drastically alter RdRP fidelity, which can be either increased or decreased depending on the viral polymerase background. In the work presented here, we extend these studies to motif D, a region that forms the outer edge of the NTP entry channel where it may act as a nucleotide sensor to trigger active site closure. Crystallography, stopped-flow kinetics, quench-flow reactions, and infectious virus studies were used to characterize 15 engineered mutations in coxsackievirus B3 polymerase. Mutations that interfere with the transport of the metal A Mg(2+) ion into the active site had only minor effects on RdRP function, but the stacking interaction between Phe(364) and Pro(357), which is absolutely conserved in enteroviral polymerases, was found to be critical for processive elongation and virus growth. Mutating Phe(364) to tryptophan resulted in a genetically stable high fidelity virus variant with significantly reduced pathogenesis in mice. The data further illustrate the importance of the palm domain movement for RdRP active site closure and demonstrate that protein engineering can be used to alter viral polymerase function and attenuate virus growth and pathogenesis.
Keywords: plus-stranded RNA virus; polymerase fidelity; protein engineering; vaccine development; viral polymerase; virology.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Steitz T. A. (1998) A mechanism for all polymerases. Nature 391, 231–232 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

