TANGO1 and Mia2/cTAGE5 (TALI) cooperate to export bulky pre-chylomicrons/VLDLs from the endoplasmic reticulum
- PMID: 27138255
- PMCID: PMC4862334
- DOI: 10.1083/jcb.201603072
TANGO1 and Mia2/cTAGE5 (TALI) cooperate to export bulky pre-chylomicrons/VLDLs from the endoplasmic reticulum
Abstract
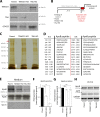
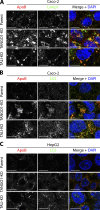
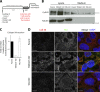
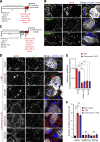
Procollagens, pre-chylomicrons, and pre-very low-density lipoproteins (pre-VLDLs) are too big to fit into conventional COPII-coated vesicles, so how are these bulky cargoes exported from the endoplasmic reticulum (ER)? We have shown that TANGO1 located at the ER exit site is necessary for procollagen export. We report a role for TANGO1 and TANGO1-like (TALI), a chimeric protein resulting from fusion of MIA2 and cTAGE5 gene products, in the export of pre-chylomicrons and pre-VLDLs from the ER. TANGO1 binds TALI, and both interact with apolipoprotein B (ApoB) and are necessary for the recruitment of ApoB-containing lipid particles to ER exit sites for their subsequent export. Although export of ApoB requires the function of both TANGO1 and TALI, the export of procollagen XII by the same cells requires only TANGO1. These findings reveal a general role for TANGO1 in the export of bulky cargoes from the ER and identify a specific requirement for TALI in assisting TANGO1 to export bulky lipid particles.
© 2016 Santos et al.
Figures




Comment in
-
Lipoprotein secretion: It takes two to TANGO.J Cell Biol. 2016 May 9;213(3):297-9. doi: 10.1083/jcb.201604084. Epub 2016 May 2. J Cell Biol. 2016. PMID: 27138249 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

