Omega-3 Fatty Acid Formulations in Cardiovascular Disease: Dietary Supplements are Not Substitutes for Prescription Products
- PMID: 27138439
- PMCID: PMC4947114
- DOI: 10.1007/s40256-016-0170-7
Omega-3 Fatty Acid Formulations in Cardiovascular Disease: Dietary Supplements are Not Substitutes for Prescription Products
Abstract
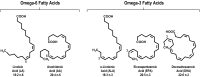
Omega-3 fatty acid products are available as prescription formulations (icosapent ethyl, omega-3-acid ethyl esters, omega-3-acid ethyl esters A, omega-3-carboxylic acids) and dietary supplements (predominantly fish oils). Most dietary supplements and all but one prescription formulation contain mixtures of the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Products containing both EPA and DHA may raise low-density lipoprotein cholesterol (LDL-C). In clinical trials, the EPA-only prescription product, icosapent ethyl, did not raise LDL-C compared with placebo. To correct a common misconception, it is important to note that omega-3 fatty acid dietary supplements are not US FDA-approved over-the-counter drugs and are not required to demonstrate safety and efficacy prior to marketing. Conversely, prescription products are supported by extensive clinical safety and efficacy investigations required for FDA approval and have active and ongoing safety monitoring programs. While omega-3 fatty acid dietary supplements may have a place in the supplementation of diet, they generally contain lower levels of EPA and DHA than prescription products and are not approved or intended to treat disease. Perhaps due to the lack of regulation of dietary supplements, EPA and DHA levels may vary widely within and between brands, and products may also contain unwanted cholesterol or fats or potentially harmful components, including toxins and oxidized fatty acids. Accordingly, omega-3 fatty acid dietary supplements should not be substituted for prescription products. Similarly, prescription products containing DHA and EPA should not be substituted for the EPA-only prescription product, as DHA may raise LDL-C and thereby complicate the management of patients with dyslipidemia.
Figures
Similar articles
-
Overview of prescription omega-3 fatty acid products for hypertriglyceridemia.Postgrad Med. 2014 Nov;126(7):7-18. doi: 10.3810/pgm.2014.11.2828. Postgrad Med. 2014. PMID: 25387209 Review.
-
Lipid effects of switching from prescription EPA+DHA (omega-3-acid ethyl esters) to prescription EPA only (icosapent ethyl) in dyslipidemic patients.Postgrad Med. 2016 Nov;128(8):859-864. doi: 10.1080/00325481.2016.1241129. Epub 2016 Oct 11. Postgrad Med. 2016. PMID: 27684412
-
Update on marine omega-3 fatty acids: management of dyslipidemia and current omega-3 treatment options.Atherosclerosis. 2013 Oct;230(2):381-9. doi: 10.1016/j.atherosclerosis.2013.07.041. Epub 2013 Jul 31. Atherosclerosis. 2013. PMID: 24075771 Review.
-
Prescription Omega-3 Fatty Acid Products and Dietary Supplements Are Not Interchangeable.Manag Care. 2016 Jan;25(1):46-52. Manag Care. 2016. PMID: 26882630 Review.
-
The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia.Lipids Health Dis. 2016 Jul 22;15(1):118. doi: 10.1186/s12944-016-0286-4. Lipids Health Dis. 2016. PMID: 27444154 Free PMC article. Review.
Cited by
-
Critical Differences Between Dietary Supplement and Prescription Omega-3 Fatty Acids: A Narrative Review.Adv Ther. 2020 Feb;37(2):656-670. doi: 10.1007/s12325-019-01211-1. Epub 2020 Jan 9. Adv Ther. 2020. PMID: 31919792 Free PMC article. Review.
-
Long-Chain Polyunsaturated Fatty Acids Effects on Cardiovascular Risk in Childhood: A Narrative Review.Nutrients. 2023 Mar 29;15(7):1661. doi: 10.3390/nu15071661. Nutrients. 2023. PMID: 37049503 Free PMC article. Review.
-
Multimodality Imaging Trials Evaluating the Impact of Omega-3 Fatty Acids on Coronary Artery Plaque Characteristics and Burden.Heart Int. 2022 Jun 30;16(1):2-11. doi: 10.17925/HI.2022.16.1.02. eCollection 2022. Heart Int. 2022. PMID: 36275355 Free PMC article. Review.
-
Oily Fish and Omega-3s Across the Life Stages: A Focus on Intakes and Future Directions.Front Nutr. 2019 Nov 12;6:165. doi: 10.3389/fnut.2019.00165. eCollection 2019. Front Nutr. 2019. PMID: 31781570 Free PMC article.
-
The Future of Lipid-lowering Therapy.J Clin Med. 2019 Jul 23;8(7):1085. doi: 10.3390/jcm8071085. J Clin Med. 2019. PMID: 31340607 Free PMC article. Review.
References
-
- Bang HO, Dyerberg J, Sinclair HM. The composition of the Eskimo food in north western Greenland. Am J Clin Nutr. 1980;33(12):2657–2661. - PubMed
-
- IUPAC-IUB Commission on Biochemical Nomenclature The nomenclature of lipids: recommendations, 1976. Eur J Biochem. 1977;79:11–21. doi: 10.1111/j.1432-1033.1977.tb11778.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

