Growth and Metastasis of Intraocular Tumors in Aged Mice
- PMID: 27138736
- PMCID: PMC4857834
- DOI: 10.1167/iovs.16-19156
Growth and Metastasis of Intraocular Tumors in Aged Mice
Abstract
Purpose: Since deterioration of the immune apparatus is closely associated with cancer, we examined the effect of aging on the growth and metastasis of intraocular melanomas in mice.
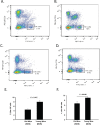
Methods: Murine B16LS9 melanoma cells were transplanted into the posterior compartment of the eye (vitreous chamber) and intraocular tumor growth and development of liver metastases were evaluated in young (8-10 weeks of age) and old (>18 months of age) mice. Liver metastases were also induced by intrasplenic injection of melanoma cells. Natural killer (NK) cells from the livers of mice harboring liver metastases were evaluated in vitro for their cytolytic activity.
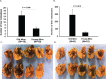
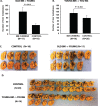
Results: Tumors grew more rapidly in the eyes of young mice than old mice, yet old mice developed significantly more liver metastases. Increased liver metastasis in old mice was evident even when melanoma cells were injected intrasplenically as a means of bypassing the influence of the ocular immunosuppressive environment. Increased liver metastases in old mice correlated with reduced cytolytic activity of liver NK cells. Lethally irradiated young mice reconstituted with bone marrow from old donors developed significantly more liver metastases than young mice reconstituted with bone marrow from young donors, indicating that bone marrow-derived cells were the root cause of the heightened development of metastases in old mice.
Conclusions: Aging affects the growth and metastasis of intraocular melanomas. Even though intraocular melanomas grow slower in old mice, the development of liver metastases is exacerbated and correlates with a reduction in liver NK cell activity in the old mouse.
Figures





Similar articles
-
NKT cells act through third party bone marrow-derived cells to suppress NK cell activity in the liver and exacerbate hepatic melanoma metastases.Int J Cancer. 2015 Sep 1;137(5):1085-94. doi: 10.1002/ijc.29480. Epub 2015 Feb 27. Int J Cancer. 2015. PMID: 25683463 Free PMC article.
-
Relationship between natural killer cell susceptibility and metastasis of human uveal melanoma cells in a murine model.Invest Ophthalmol Vis Sci. 1995 Feb;36(2):435-41. Invest Ophthalmol Vis Sci. 1995. PMID: 7843912
-
NKT cell exacerbation of liver metastases arising from melanomas transplanted into either the eyes or spleens of mice.Invest Ophthalmol Vis Sci. 2011 May 11;52(6):3094-102. doi: 10.1167/iovs.10-7067. Invest Ophthalmol Vis Sci. 2011. PMID: 21330669 Free PMC article.
-
[Metastatic uveal melanoma. Development of neoadjuvant treatment in animal experiments].Ophthalmologe. 2001 Aug;98(8):761-5. doi: 10.1007/s003470170084. Ophthalmologe. 2001. PMID: 11552416 Review. German.
-
Screening for uveal melanoma metastasis. Literature review.Bull Soc Belge Ophtalmol. 2004;(294):13-22. Bull Soc Belge Ophtalmol. 2004. PMID: 15682915 Review.
Cited by
-
Animal Models of Uveal Melanoma.Ann Eye Sci. 2022 Mar;7:7. doi: 10.21037/aes-21-30. Epub 2022 Mar 15. Ann Eye Sci. 2022. PMID: 35350473 Free PMC article.
-
The complex interplay between aging and cancer.Nat Aging. 2025 Mar;5(3):350-365. doi: 10.1038/s43587-025-00827-z. Epub 2025 Mar 4. Nat Aging. 2025. PMID: 40038418 Review.
-
Defective FasL expression is associated with increased resistance to melanoma liver metastases and enhanced natural killer cell activity.Melanoma Res. 2019 Aug;29(4):401-412. doi: 10.1097/CMR.0000000000000614. Melanoma Res. 2019. PMID: 30932943 Free PMC article.
-
Modelling physical resilience in ageing mice.Mech Ageing Dev. 2019 Jan;177:91-102. doi: 10.1016/j.mad.2018.10.001. Epub 2018 Oct 2. Mech Ageing Dev. 2019. PMID: 30290161 Free PMC article. Review.
-
Liver resection for colorectal liver-limited metastases in elderly patients: a propensity score matching analysis.World J Surg Oncol. 2020 Oct 24;18(1):275. doi: 10.1186/s12957-020-02055-8. World J Surg Oncol. 2020. PMID: 33099304 Free PMC article.
References
-
- Mocchegiani E,, Malavolta M. NK, and NKT cell functions in immunosenescence. Aging Cell. 2004; 3: 177–184. - PubMed
-
- Provinciali M,, Barucca A,, Cardelli M,, Marchegiani F,, Pierpaoli E. Inflammation aging, and cancer vaccines. Biogerontology. 2010; 11: 615–626. - PubMed
-
- Taub DD,, Longo DL. Insights into thymic aging and regeneration. Immunol Rev. 2005; 205: 72–93. - PubMed
-
- Song L,, Kim YH,, Chopra RK,, et al. Age-related effects in T cell activation and proliferation. Exp Gerontol. 1993; 28: 313–321. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

