Intensified vmPFC surveillance over PTSS under perturbed microRNA-608/AChE interaction
- PMID: 27138800
- PMCID: PMC5070052
- DOI: 10.1038/tp.2016.70
Intensified vmPFC surveillance over PTSS under perturbed microRNA-608/AChE interaction
Abstract
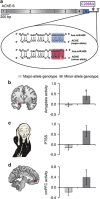
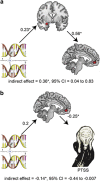
Trauma causes variable risk of posttraumatic stress symptoms (PTSS) owing to yet-unknown genome-neuronal interactions. Here, we report co-intensified amygdala and ventromedial prefrontal cortex (vmPFC) emotional responses that may overcome PTSS in individuals with the single-nucleotide polymorphism (SNP) rs17228616 in the acetylcholinesterase (AChE) gene. We have recently shown that in individuals with the minor rs17228616 allele, this SNP interrupts AChE suppression by microRNA (miRNA)-608, leading to cortical elevation of brain AChE and reduced cortisol and the miRNA-608 target GABAergic modulator CDC42, all stress-associated. To examine whether this SNP has effects on PTSS and threat-related brain circuits, we exposed 76 healthy Israel Defense Forces soldiers who experienced chronic military stress to a functional magnetic resonance imaging task of emotional and neutral visual stimuli. Minor allele individuals predictably reacted to emotional stimuli by hyperactivated amygdala, a hallmark of PTSS and a predisposing factor of posttraumatic stress disorder (PTSD). Despite this, minor allele individuals showed no difference in PTSS levels. Mediation analyses indicated that the potentiated amygdala reactivity in minor allele soldiers promoted enhanced vmPFC recruitment that was associated with their limited PTSS. Furthermore, we found interrelated expression levels of several miRNA-608 targets including CD44, CDC42 and interleukin 6 in human amygdala samples (N=7). Our findings suggest that miRNA-608/AChE interaction is involved in the threat circuitry and PTSS and support a model where greater vmPFC regulatory activity compensates for amygdala hyperactivation in minor allele individuals to neutralize their PTSS susceptibility.
Figures


Similar articles
-
Impact of posttraumatic stress symptom dimensions on amygdala reactivity to emotional faces.Prog Neuropsychopharmacol Biol Psychiatry. 2017 Oct 3;79(Pt B):401-407. doi: 10.1016/j.pnpbp.2017.07.021. Epub 2017 Jul 26. Prog Neuropsychopharmacol Biol Psychiatry. 2017. PMID: 28756011 Free PMC article.
-
Prefrontal-Amygdala Dysregulation to Threat in Pediatric Posttraumatic Stress Disorder.Neuropsychopharmacology. 2016 Feb;41(3):822-31. doi: 10.1038/npp.2015.209. Epub 2015 Jul 14. Neuropsychopharmacology. 2016. PMID: 26171717 Free PMC article.
-
The suppression of brain activation in post-deployment military personnel with posttraumatic stress symptoms.Brain Imaging Behav. 2015 Sep;9(3):513-26. doi: 10.1007/s11682-015-9376-6. Brain Imaging Behav. 2015. PMID: 25875014
-
[Posttraumatic stress disorder (PTSD) as a consequence of the interaction between an individual genetic susceptibility, a traumatogenic event and a social context].Encephale. 2012 Oct;38(5):373-80. doi: 10.1016/j.encep.2011.12.003. Epub 2012 Jan 24. Encephale. 2012. PMID: 23062450 Review. French.
-
Amygdala, medial prefrontal cortex, and hippocampal function in PTSD.Ann N Y Acad Sci. 2006 Jul;1071:67-79. doi: 10.1196/annals.1364.007. Ann N Y Acad Sci. 2006. PMID: 16891563 Review.
Cited by
-
Epigenetic Alterations in Post-Traumatic Stress Disorder: Comprehensive Review of Molecular Markers.Complex Psychiatry. 2024 Nov 18;10(1-4):71-107. doi: 10.1159/000541822. eCollection 2024 Jan-Dec. Complex Psychiatry. 2024. PMID: 39564465 Free PMC article. Review.
-
Ribosomal protein L24 mediates mammalian microRNA processing in an evolutionarily conserved manner.Cell Mol Life Sci. 2024 Jan 23;81(1):55. doi: 10.1007/s00018-023-05088-w. Cell Mol Life Sci. 2024. PMID: 38261097 Free PMC article.
-
Cholinergic blockade of neuroinflammation: from tissue to RNA regulators.Neuronal Signal. 2022 Feb 11;6(1):NS20210035. doi: 10.1042/NS20210035. eCollection 2022 Apr. Neuronal Signal. 2022. PMID: 35211331 Free PMC article. Review.
-
Personalized genetics of the cholinergic blockade of neuroinflammation.J Neurochem. 2017 Aug;142 Suppl 2(Suppl Suppl 2):178-187. doi: 10.1111/jnc.13928. Epub 2017 Mar 21. J Neurochem. 2017. PMID: 28326544 Free PMC article. Review.
-
Neuronal-expressed microRNA-targeted pseudogenes compete with coding genes in the human brain.Transl Psychiatry. 2017 Aug 8;7(8):e1199. doi: 10.1038/tp.2017.163. Transl Psychiatry. 2017. PMID: 28786976 Free PMC article.
References
-
- Lin T, Vaisvaser S, Fruchter E, Admon R, Wald I, Pine DS et al. A neurobehavioral account for individual differences in resilience to chronic military stress. Psychol Med 2015; 45: 1011–1023. - PubMed
-
- Taylor MK, Sausen KP, Potterat EG, Mujica-Parodi LR, Reis JP, Markham AE et al. Stressful military training: endocrine reactivity, performance, and psychological impact. Aviat Space Environ Med 2007; 78: 1143–1149. - PubMed
-
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Psychiatry 1995; 52: 1048–1060. - PubMed
-
- Cukor J, Wyka K, Jayasinghe N, Difede J. The nature and course of subthreshold PTSD. J Anxiety Disord 2010; 24: 918–923. - PubMed
-
- Grasso DJ, Cohen LH, Moser JS, Hajcak G, Foa EB, Simons RF. Seeing the silver lining: potential benefits of trauma exposure in college students. Anxiety Stress Coping 2012; 25: 117–136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

