Wnt pathway activation by ADP-ribosylation
- PMID: 27138857
- PMCID: PMC4857404
- DOI: 10.1038/ncomms11430
Wnt pathway activation by ADP-ribosylation
Abstract
Wnt/β-catenin signalling directs fundamental processes during metazoan development and can be aberrantly activated in cancer. Wnt stimulation induces the recruitment of the scaffold protein Axin from an inhibitory destruction complex to a stimulatory signalosome. Here we analyse the early effects of Wnt on Axin and find that the ADP-ribose polymerase Tankyrase (Tnks)--known to target Axin for proteolysis-regulates Axin's rapid transition following Wnt stimulation. We demonstrate that the pool of ADP-ribosylated Axin, which is degraded under basal conditions, increases immediately following Wnt stimulation in both Drosophila and human cells. ADP-ribosylation of Axin enhances its interaction with the Wnt co-receptor LRP6, an essential step in signalosome assembly. We suggest that in addition to controlling Axin levels, Tnks-dependent ADP-ribosylation promotes the reprogramming of Axin following Wnt stimulation; and propose that Tnks inhibition blocks Wnt signalling not only by increasing destruction complex activity, but also by impeding signalosome assembly.
Figures



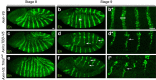
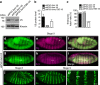
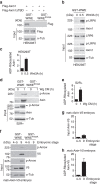

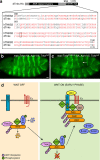
References
-
- Clevers H. & Nusse R. Wnt/beta-catenin signaling and disease. Cell 149, 1192–1205 (2012). - PubMed
-
- Behrens J. et al.. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science 280, 596–599 (1998). - PubMed
-
- Kimelman D. & Xu W. beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene 25, 7482–7491 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

