Characterisation of Population Pharmacokinetics and Endogenous Follicle-Stimulating Hormone (FSH) Levels After Multiple Dosing of a Recombinant Human FSH (FE 999049) in Healthy Women
- PMID: 27139012
- PMCID: PMC4875921
- DOI: 10.1007/s40268-016-0126-z
Characterisation of Population Pharmacokinetics and Endogenous Follicle-Stimulating Hormone (FSH) Levels After Multiple Dosing of a Recombinant Human FSH (FE 999049) in Healthy Women
Abstract
Objective: The aim of this study was to characterise the population pharmacokinetics of FE 999049, a novel recombinant human follicle-stimulating hormone (FSH), after multiple dosing in healthy women, taking into account endogenous FSH levels and the reproductive hormone dynamics.
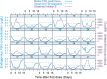
Methods: Longitudinal measurements of FSH, luteinising hormone, progesterone, estradiol, and inhibin B levels were collected after repeated subcutaneous dosing with 225 IU of FE 999049 in 24 gonadotropin downregulated healthy women. The FSH data were described using nonlinear mixed-effects modelling.
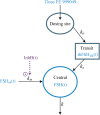
Results: The measured FSH levels were modelled as a sum of endogenous FSH and FE 999049. The FE 999049 population pharmacokinetics were best described using a one-compartment model with first-order absorption and elimination, and a transit model for delayed absorption. The apparent clearance and volume of distribution increased with body weight in accordance with an allometrically scaled power exponent of 0.75 and 1, respectively. Endogenous FSH levels were lower in individuals with higher progesterone levels at baseline and were further suppressed over time with increasing inhibin B levels.
Conclusions: This characterisation of FE 999049 population pharmacokinetics after repeated dosing is in line with previous findings after single-dose administration. The results provide a basis for study design and data evaluation in the future development of recombinant FSH products, and show it can be of importance to account for endogenous FSH levels and its variation over time for accurate estimation of exogenously administered FSH pharmacokinetic parameters. Thus, correcting FSH concentrations by the observed endogenous FSH baseline value at all time points may be incorrect.
Figures



References
-
- Beal S, Sheiner LB, Boeckmann A, Bauer RJ. NONMEM user’s guides (1989-2011) Ellicott City: Icon Development Solutions; 2011.
-
- R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2010.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

