Tackling aspecific side reactions during histone propionylation: The promise of reversing overpropionylation
- PMID: 27139031
- PMCID: PMC5096241
- DOI: 10.1002/pmic.201600045
Tackling aspecific side reactions during histone propionylation: The promise of reversing overpropionylation
Abstract
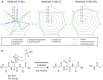
Histone proteins are essential elements for DNA packaging. Moreover, the PTMs that are extremely abundant on these proteins, contribute in modeling chromatin structure and recruiting enzymes involved in gene regulation, DNA repair and chromosome condensation. This fundamental aspect, together with the epigenetic inheritance of histone PTMs, underlines the importance of having biochemical techniques for their characterization. Over the past two decades, significant improvements in mass accuracy and resolution of mass spectrometers have made LC-coupled MS the strategy of choice for accurate identification and quantification of protein PTMs. Nevertheless, in previous work we disclosed the limitations and biases of the most widely adopted sample preparation protocols for histone propionylation, required prior to bottom-up MS analysis. In this work, however, we put forward a new specific and efficient propionylation strategy by means of propionic anhydride. In this method, aspecific overpropionylation at serine (S), threonine (T) and tyrosine (Y) is reversed by adding hydroxylamine (HA). We recommend using this method for future analysis of histones through bottom-up MS.
Keywords: Histone; Mass spectrometry; Method optimization; Propionylation; Technology.
© 2016 The Authors. Proteomics Published by Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

References
-
- Sidoli, S. , Garcia, B. A. , Properly reading the histone code by MS‐based proteomics. Proteomics 2015, 15, 2901–2902. - PubMed
-
- Abello, N. , Kerstjens, H. A. M. , Postma, D. S. , Bischoff, R. , Selective acylation of primary amines in peptides and proteins. J. Proteome Res. 2007, 6, 4770–4776. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

