Antifungal Mechanism of Action of Lactoferrin: Identification of H+-ATPase (P3A-Type) as a New Apoptotic-Cell Membrane Receptor
- PMID: 27139463
- PMCID: PMC4914641
- DOI: 10.1128/AAC.03130-15
Antifungal Mechanism of Action of Lactoferrin: Identification of H+-ATPase (P3A-Type) as a New Apoptotic-Cell Membrane Receptor
Abstract
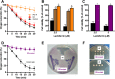
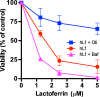
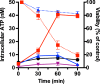
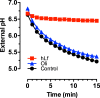
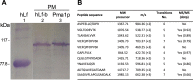
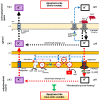
Human lactoferrin (hLf) is a protein of the innate immune system which induces an apoptotic-like process in yeast. Determination of the susceptibility to lactoferrin of several yeast species under different metabolic conditions, respiratory activity, cytoplasmic ATP levels, and external medium acidification mediated by glucose assays suggested plasma membrane Pma1p (P3A-type ATPase) as the hLf molecular target. The inhibition of plasma membrane ATPase activity by hLf and the identification of Pma1p as the hLf-binding membrane protein confirmed the previous physiological evidence. Consistent with this, cytoplasmic ATP levels progressively increased in hLf-treated Candida albicans cells. However, oligomycin, a specific inhibitor of the mitochondrial F-type ATPase proton pump (mtATPase), abrogated the antifungal activity of hLf, indicating a crucial role for mtATPase in the apoptotic process. We suggest that lactoferrin targeted plasma membrane Pma1p H(+)-ATPase, perturbing the cytoplasmic ion homeostasis (i.e., cytoplasmic H(+) accumulation and subsequent K(+) efflux) and inducing a lethal mitochondrial dysfunction. This initial event involved a normal mitochondrial ATP synthase activity responsible for both the ATP increment and subsequent hypothetical mitochondrial proton flooding process. We conclude that human lactoferrin inhibited Pma1p H(+)-ATPase, inducing an apoptotic-like process in metabolically active yeast. Involvement of mitochondrial H(+)-ATPase (nonreverted) was essential for the progress of this programmed cell death in which the ionic homeostasis perturbation seems to precede classical nonionic apoptotic events.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Choi H, Lee W, Lee DG. 2013. A new concept on mechanism of antimicrobial peptides: apoptosis induction, p 775–785. In Ḿendez-Vilas A. (ed), Microbial pathogens and strategies for combating them: science, technology and education, vol 2 Formatex Research Center, Badajoz, Spain.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

