Anti-infective Activity of 2-Cyano-3-Acrylamide Inhibitors with Improved Drug-Like Properties against Two Intracellular Pathogens
- PMID: 27139470
- PMCID: PMC4914689
- DOI: 10.1128/AAC.03021-15
Anti-infective Activity of 2-Cyano-3-Acrylamide Inhibitors with Improved Drug-Like Properties against Two Intracellular Pathogens
Abstract
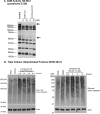
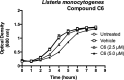
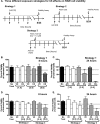
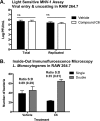
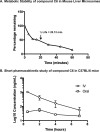
Due to the rise of antibiotic resistance and the small number of effective antiviral drugs, new approaches for treating infectious diseases are urgently needed. Identifying targets for host-based therapies represents an emerging strategy for drug discovery. The ubiquitin-proteasome system is a central mode of signaling in the eukaryotic cell and may be a promising target for therapies that bolster the host's ability to control infection. Deubiquitinase (DUB) enzymes are key regulators of the host inflammatory response, and we previously demonstrated that a selective DUB inhibitor and its derivative promote anti-infective activities in host cells. To find compounds with anti-infective efficacy but improved toxicity profiles, we tested a library of predominantly 2-cyano-3-acrylamide small-molecule DUB inhibitors for anti-infective activity in macrophages against two intracellular pathogens: murine norovirus (MNV) and Listeria monocytogenes We identified compound C6, which inhibited DUB activity in human and murine cells and reduced intracellular replication of both pathogens with minimal toxicity in cell culture. Treatment with C6 did not significantly affect the ability of macrophages to internalize virus, suggesting that the anti-infective activity interferes with postentry stages of the MNV life cycle. Metabolic stability and pharmacokinetic assays showed that C6 has a half-life in mouse liver microsomes of ∼20 min and has a half-life of approximately 4 h in mice when administered intravenously. Our results provide a framework for targeting the host ubiquitin system in the development of host-based therapies for infectious disease. Compound C6 represents a promising tool with which to elucidate the role of DUBs in the macrophage response to infection.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
Small molecule deubiquitinase inhibitors promote macrophage anti-infective capacity.PLoS One. 2014 Aug 5;9(8):e104096. doi: 10.1371/journal.pone.0104096. eCollection 2014. PLoS One. 2014. PMID: 25093325 Free PMC article.
-
Antiviral activity of a small molecule deubiquitinase inhibitor occurs via induction of the unfolded protein response.PLoS Pathog. 2012;8(7):e1002783. doi: 10.1371/journal.ppat.1002783. Epub 2012 Jul 5. PLoS Pathog. 2012. PMID: 22792064 Free PMC article.
-
Chemical derivatives of a small molecule deubiquitinase inhibitor have antiviral activity against several RNA viruses.PLoS One. 2014 Apr 10;9(4):e94491. doi: 10.1371/journal.pone.0094491. eCollection 2014. PLoS One. 2014. PMID: 24722666 Free PMC article.
-
Current tools for norovirus drug discovery.Expert Opin Drug Discov. 2016 Jun;11(6):529-41. doi: 10.1080/17460441.2016.1178231. Epub 2016 May 2. Expert Opin Drug Discov. 2016. PMID: 27108716 Free PMC article. Review.
-
Manipulation of viral infection by deubiquitinating enzymes: new players in host-virus interactions.Future Microbiol. 2016 Oct;11:1435-1446. doi: 10.2217/fmb-2016-0091. Epub 2016 Oct 27. Future Microbiol. 2016. PMID: 27785925 Review.
Cited by
-
Perturbation of ubiquitin homeostasis promotes macrophage oxidative defenses.Sci Rep. 2019 Jul 15;9(1):10245. doi: 10.1038/s41598-019-46526-9. Sci Rep. 2019. PMID: 31308397 Free PMC article.
-
Therapeutics and Immunoprophylaxis Against Noroviruses and Rotaviruses: The Past, Present, and Future.Curr Drug Metab. 2018;19(3):170-191. doi: 10.2174/1389200218666170912161449. Curr Drug Metab. 2018. PMID: 28901254 Free PMC article. Review.
-
Impaired K48-polyubiquitination downmodulates mouse norovirus propagation.Front Cell Infect Microbiol. 2025 May 6;15:1530166. doi: 10.3389/fcimb.2025.1530166. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40395509 Free PMC article.
-
The Role of Deubiquitinases in Virus Replication and Host Innate Immune Response.Front Microbiol. 2022 Feb 24;13:839624. doi: 10.3389/fmicb.2022.839624. eCollection 2022. Front Microbiol. 2022. PMID: 35283827 Free PMC article. Review.
-
Viral deubiquitinating proteases and the promising strategies of their inhibition.Virus Res. 2024 Jun;344:199368. doi: 10.1016/j.virusres.2024.199368. Epub 2024 Apr 11. Virus Res. 2024. PMID: 38588924 Free PMC article. Review.
References
-
- World Health Organization. 2014. Antimicrobial resistance: global report on surveillance 2014. World Health Organization, Geneva, Switzerland: http://www.who.int/drugresistance/documents/surveillancereport/en/.
-
- Basarab GS, Kern GH, McNulty J, Mueller JP, Lawrence K, Vishwanathan K, Alm RA, Barvian K, Doig P, Galullo V, Gardner H, Gowravaram M, Huband M, Kimzey A, Morningstar M, Kutschke A, Lahiri SD, Perros M, Singh R, Schuck VJ, Tommasi R, Walkup G, Newman JV. 2015. Responding to the challenge of untreatable gonorrhea: ETX0914, a first-in-class agent with a distinct mechanism-of-action against bacterial type II topoisomerases. Sci Rep 5:11827. doi:10.1038/srep11827. - DOI - PMC - PubMed
-
- Mather AE, Reid SW, Maskell DJ, Parkhill J, Fookes MC, Harris SR, Brown DJ, Coia JE, Mulvey MR, Gilmour MW, Petrovska L, de Pinna E, Kuroda M, Akiba M, Izumiya H, Connor TR, Suchard MA, Lemey P, Mellor DJ, Haydon DT, Thomson NR. 2013. Distinguishable epidemics of multidrug-resistant Salmonella Typhimurium DT104 in different hosts. Science 341:1514–1517. doi:10.1126/science.1240578. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

