Characteristics and Outcomes of Complicated Intra-abdominal Infections Involving Pseudomonas aeruginosa from a Randomized, Double-Blind, Phase 3 Ceftolozane-Tazobactam Study
- PMID: 27139477
- PMCID: PMC4914617
- DOI: 10.1128/AAC.03074-15
Characteristics and Outcomes of Complicated Intra-abdominal Infections Involving Pseudomonas aeruginosa from a Randomized, Double-Blind, Phase 3 Ceftolozane-Tazobactam Study
Abstract
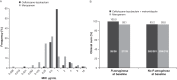
Ceftolozane-tazobactam is active against Gram-negative pathogens, including multidrug-resistant Pseudomonas aeruginosa In a subgroup analysis of patients with complicated intra-abdominal infections (cIAIs) involving P. aeruginosa from a phase 3 program, ceftolozane-tazobactam demonstrated potent in vitro activity against P. aeruginosa Clinical cure in the microbiologically evaluable population was 100% (26/26) for ceftolozane-tazobactam plus metronidazole and 93.1% (27/29) for meropenem. These findings support the use of ceftolozane-tazobactam in the management of cIAI when P. aeruginosa is suspected or confirmed. (This study has been registered at ClinicalTrials.gov under registration no. NCT01445665 and NCT01445678.).
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- Sartelli M, Catena F, Ansaloni L, Coccolini F, Corbella D, Moore EE, Malangoni M, Velmahos G, Coimbra R, Koike K, Leppaniemi A, Biffl W, Balogh Z, Bendinelli C, Gupta S, Kluger Y, Agresta F, Di Saverio S, Tugnoli G, Jovine E, Ordonez CA, Whelan JF, Fraga GP, Gomes CA, Pereira GA, Yuan KC, Bala M, Peev MP, Ben-Ishay O, Cui Y, Marwah S, Zachariah S, Wani I, Rangarajan M, Sakakushev B, Kong V, Ahmed A, Abbas A, Gonsaga RA, Guercioni G, Vettoretto N, Poiasina E, Diaz-Nieto R, Massalou D, Skrovina M, Gerych I, Augustin G, Kenig J, Khokha V, Trana C, Kok KY, Mefire AC, Lee JG, Hong SK, Lohse HA, Ghnnam W, Verni A, Lohsiriwat V, Siribumrungwong B, El Zalabany T, Tavares A, Baiocchi G, Das K, Jarry J, Zida M, Sato N, Murata K, Shoko T, Irahara T, Hamedelneel AO, Naidoo N, Adesunkanmi AR, Kobe Y, Ishii W, Oka K, Izawa Y, Hamid H, Khan I, Attri A, Sharma R, Sanjuan J, Badiel M, Barnabe R. 2014. Complicated intra-abdominal infections worldwide: the definitive data of the CIAOW study. World J Emerg Surg 9:37. doi:10.1186/1749-7922-9-37. - DOI - PMC - PubMed
-
- Center for Disease Dynamics Economics & Policy. 2013. ResistanceMap database: The Surveillance Network. USA Center for Disease Dynamics, Economics & Policy, Washington, DC: http://www.cddep.org/map Accessed 17 March 2016.
-
- European Centre for Disease Prevention and Control. 2013. Antimicrobial resistance surveillance in Europe 2013. Annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). http://ecdc.europa.eu/en/publications/Publications/antimicrobial-resista... Accessed 17 March 2016.
-
- Merck & Co., Inc. 2015. Zerbaxa prescribing information. Merck & Co., Inc., Whitehouse Station, NJ.
-
- Sader HS, Rhomberg PR, Farrell DJ, Jones RN. 2011. Antimicrobial activity of CXA-101, a novel cephalosporin tested in combination with tazobactam against Enterobacteriaceae, Pseudomonas aeruginosa, and Bacteroides fragilis strains having various resistance phenotypes. Antimicrob Agents Chemother 55:2390–2394. doi:10.1128/AAC.01737-10. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

