Quantitative proteomics of the tobacco pollen tube secretome identifies novel pollen tube guidance proteins important for fertilization
- PMID: 27139692
- PMCID: PMC4853860
- DOI: 10.1186/s13059-016-0928-x
Quantitative proteomics of the tobacco pollen tube secretome identifies novel pollen tube guidance proteins important for fertilization
Abstract
Background: As in animals, cell-cell communication plays a pivotal role in male-female recognition during plant sexual reproduction. Prelaid peptides secreted from the female reproductive tissues guide pollen tubes towards ovules for fertilization. However, the elaborate mechanisms for this dialogue have remained elusive, particularly from the male perspective.
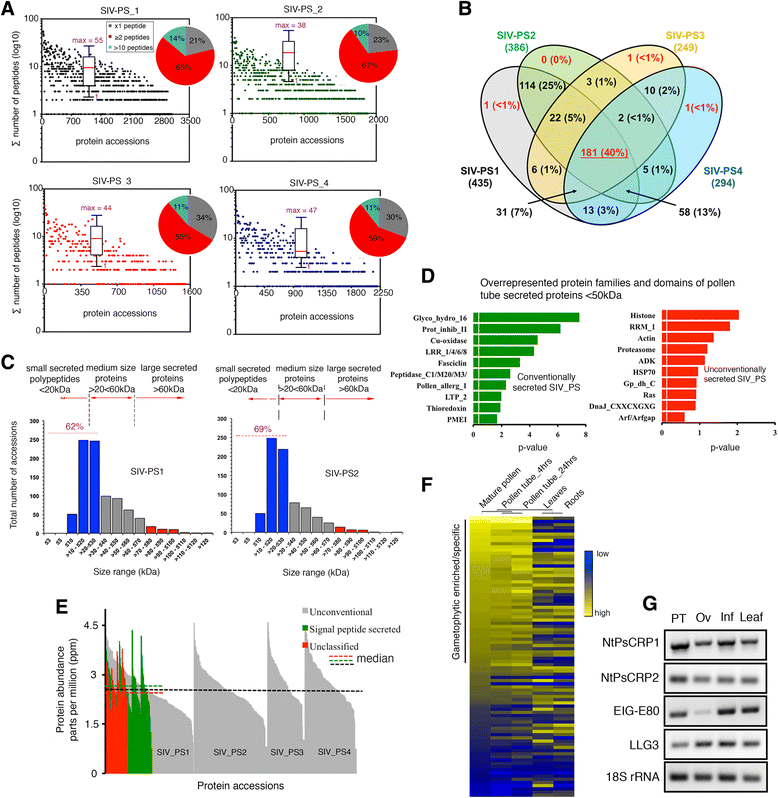
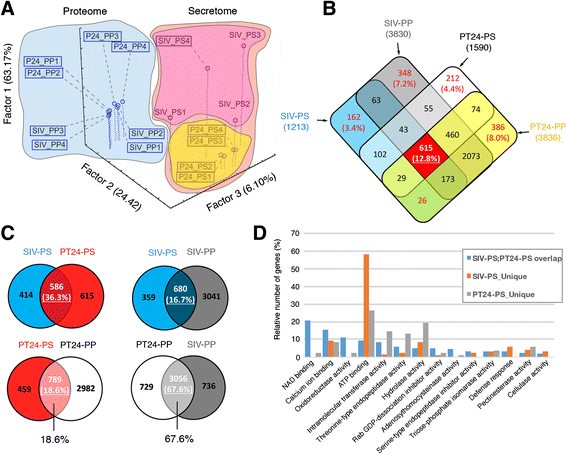
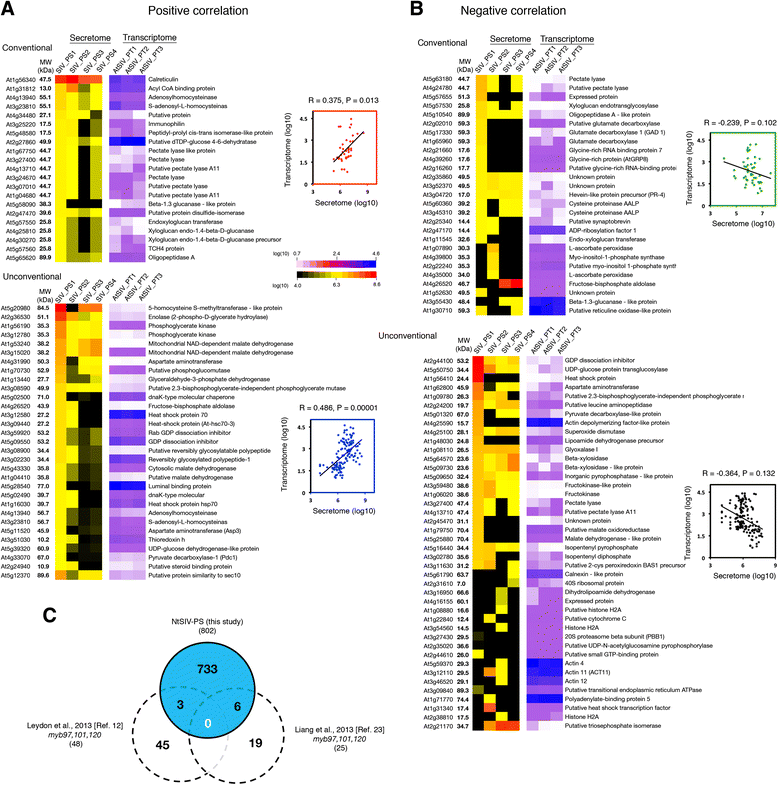
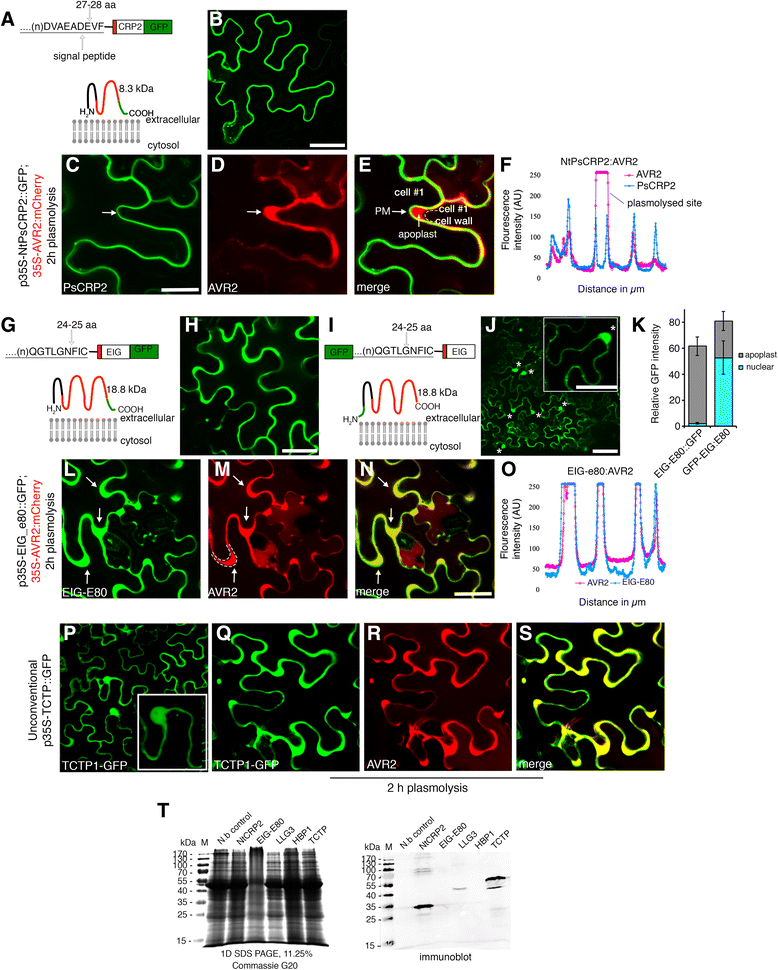
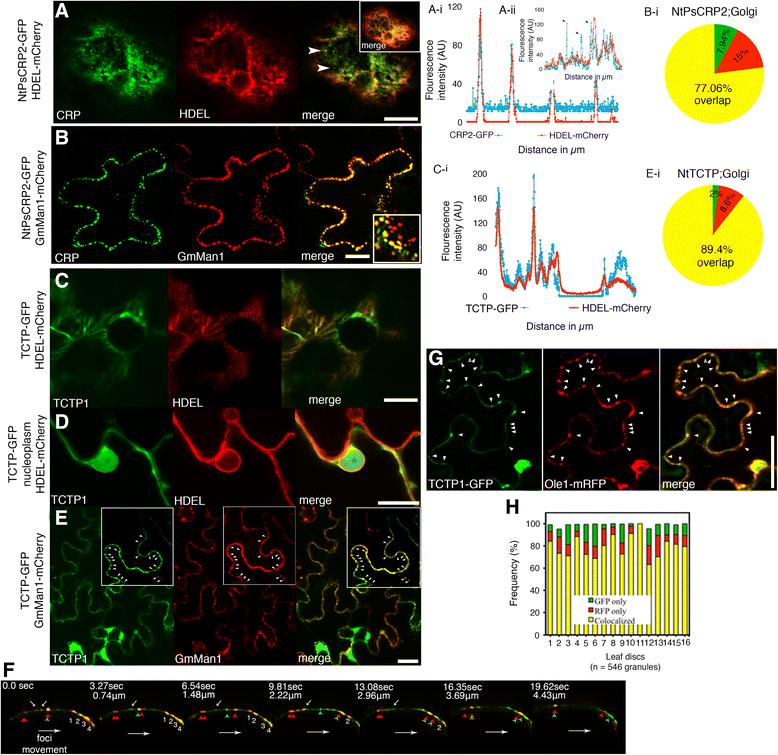
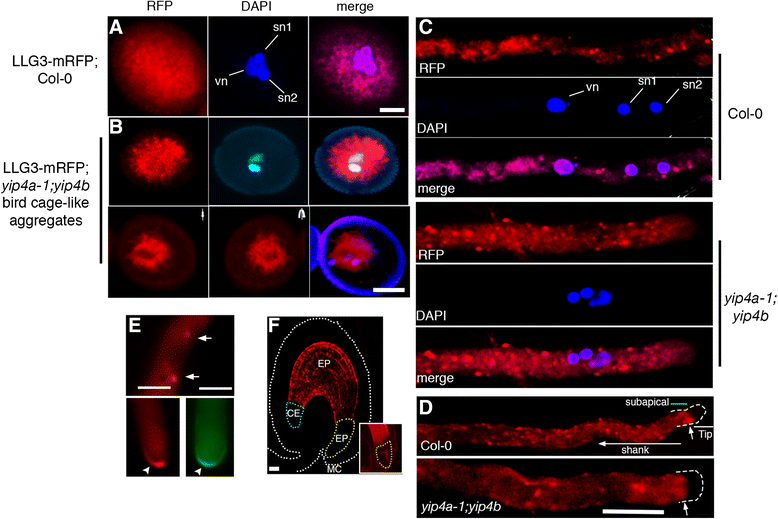
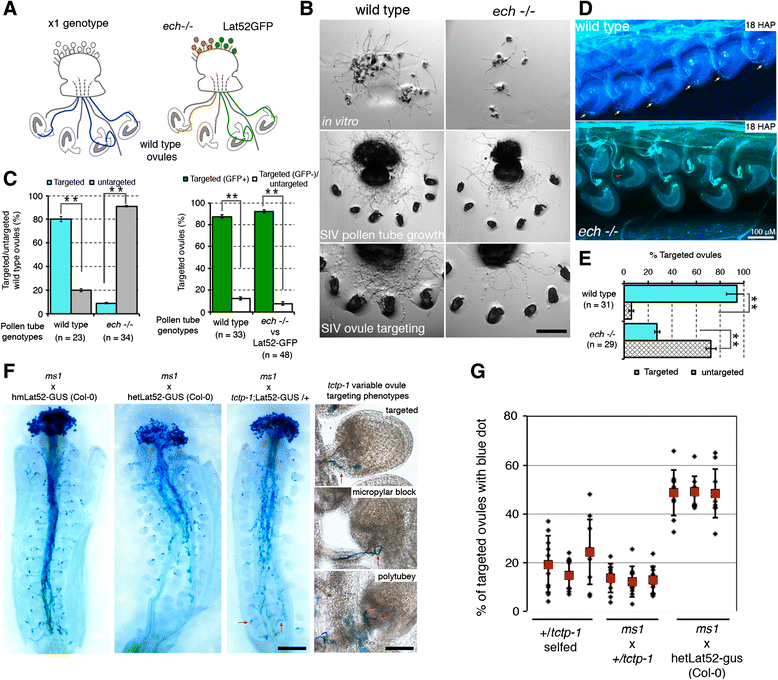
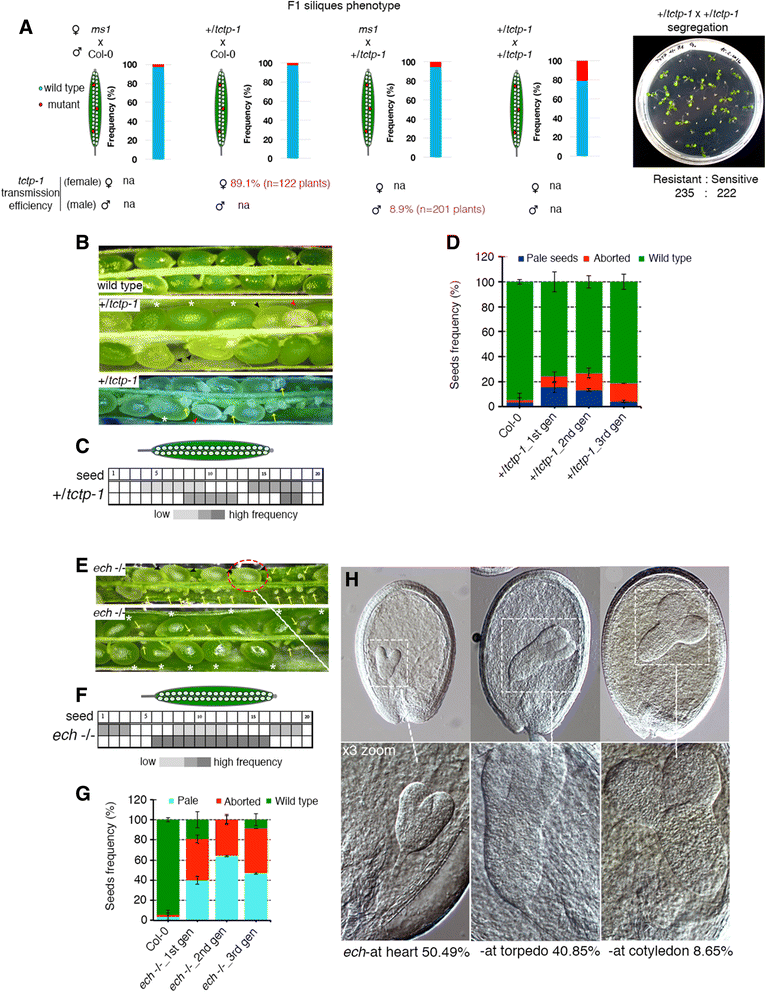
Results: We performed genome-wide quantitative liquid chromatography-tandem mass spectrometry analysis of a pistil-stimulated pollen tube secretome and identified 801 pollen tube-secreted proteins. Interestingly, in silico analysis reveals that the pollen tube secretome is dominated by proteins that are secreted unconventionally, representing 57 % of the total secretome. In support, we show that an unconventionally secreted protein, translationally controlled tumor protein, is secreted to the apoplast. Remarkably, we discovered that this protein could be secreted by infiltrating through the initial phases of the conventional secretory pathway and could reach the apoplast via exosomes, as demonstrated by co-localization with Oleisin1 exosome marker. We demonstrate that translationally controlled tumor protein-knockdown Arabidopsis thaliana plants produce pollen tubes that navigate poorly to the target ovule and that the mutant allele is poorly transmitted through the male. Further, we show that regulators of the endoplasmic reticulum-trans-Golgi network protein secretory pathway control secretion of Nicotiana tabacum Pollen tube-secreted cysteine-rich protein 2 and Lorelei-like GPI-anchor protein 3 and that a regulator of endoplasmic reticulum-trans-Golgi protein translocation is essential for pollen tube growth, pollen tube guidance and ovule-targeting competence.
Conclusions: This work, the first study on the pollen tube secretome, identifies novel genome-wide pollen tube-secreted proteins with potential functions in pollen tube guidance towards ovules for sexual reproduction. Functional analysis highlights a potential mechanism for unconventional secretion of pollen tube proteins and reveals likely regulators of conventional pollen tube protein secretion. The association of pollen tube-secreted proteins with marker proteins shown to be secreted via exosomes in other species suggests exosome secretion is a possible mechanism for cell-cell communication between the pollen tube and female reproductive cells.
Keywords: Cell-cell signaling; Double fertilization; Pollen tube guidance; Protein secretion.
Figures

Comment in
-
Maybe she's NOT the boss: male-female crosstalk during sexual plant reproduction.Genome Biol. 2016 May 9;17:96. doi: 10.1186/s13059-016-0972-6. Genome Biol. 2016. PMID: 27159978 Free PMC article.
References
-
- Maheshwari P. An introduction to embryology of angiosperms. New York: McGraw-Hill; 1950.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

