Amyloid-β plaque deposition measured using propagation-based X-ray phase contrast CT imaging
- PMID: 27140162
- PMCID: PMC5315008
- DOI: 10.1107/S1600577516004045
Amyloid-β plaque deposition measured using propagation-based X-ray phase contrast CT imaging
Abstract
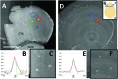
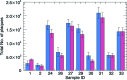
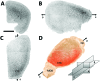
Amyloid beta accumulation into insoluble plaques (Aβp) is known to play a significant role in the pathological process in Alzheimer's disease (AD). The presence of Aβp is also one of the neuropathological hallmarks for the disease. AD final diagnosis is generally acknowledged after the evaluation of Aβp deposition in the brain. Insoluble Aβp accumulation may also concur to cause AD as postulated in the so-called amyloid hypothesis. Therefore, the visualization, evaluation and quantification of Aβp are nowadays the keys for a better understanding of the disease, which may point to a possible cure for AD in the near future. Synchrotron-based X-ray phase contrast (XPC) has been demonstrated as the only imaging method that can retrieve the Aβp signal with high spatial resolution (up to 10 µm), high sensitivity and three-dimensional information at the same time. Although at the moment XPC is suitable for ex vivo samples only, it may develop into an alternative to positron emission tomography and magnetic resonance imaging in Aβp imaging. In this contribution the possibility of using synchrotron-based X-ray phase propagation computed tomography to visualize and measure Aβp on mouse brains is presented. A careful setup optimization for this application leads to a significant improvement of spatial resolution (∼1 µm), data acquisition speed (five times faster), X-ray dose (five times lower) and setup complexity, without a substantial loss in sensitivity when compared with the classic implementation of grating-based X-ray interferometry.
Keywords: Alzheimer's disease; X-ray CT; amyloid plaques; brain imaging; phase contrast.
Figures


References
-
- Astolfo, A., Arfelli, F., Schultke, E., James, S., Mancini, L. & Menk, R. H. (2013). Nanoscale, 5, 3337–3345. - PubMed
-
- Beltran, M. A., Paganin, D. M., Siu, K. K. W., Fouras, A., Hooper, S. B., Reser, D. H. & Kitchen, M. J. (2011). Phys. Med. Biol. 56, 7353–7369. - PubMed
-
- Braak, H. & Braak, E. (1991). Acta Neuropathol. 82, 239–259. - PubMed
-
- Castelli, E. et al. (2011). Radiology, 259, 684–694. - PubMed
-
- Chen, R., Liu, P., Xiao, T. & Xu, L. X. (2014). Adv. Mater. 26, 7688–7691. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

