Alternatively activated macrophages determine repair of the infarcted adult murine heart
- PMID: 27140396
- PMCID: PMC4887176
- DOI: 10.1172/JCI85782
Alternatively activated macrophages determine repair of the infarcted adult murine heart
Abstract
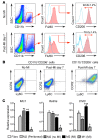
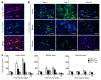
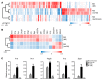
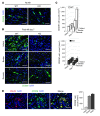
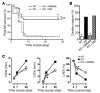
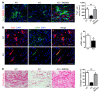
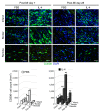
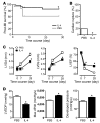
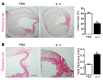
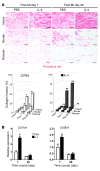
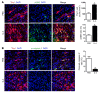
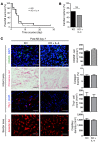
Alternatively activated (also known as M2) macrophages are involved in the repair of various types of organs. However, the contribution of M2 macrophages to cardiac repair after myocardial infarction (MI) remains to be fully characterized. Here, we identified CD206+F4/80+CD11b+ M2-like macrophages in the murine heart and demonstrated that this cell population predominantly increases in the infarct area and exhibits strengthened reparative abilities after MI. We evaluated mice lacking the kinase TRIB1 (Trib1-/-), which exhibit a selective depletion of M2 macrophages after MI. Compared with control animals, Trib1-/- mice had a catastrophic prognosis, with frequent cardiac rupture, as the result of markedly reduced collagen fibril formation in the infarct area due to impaired fibroblast activation. The decreased tissue repair observed in Trib1-/- mice was entirely rescued by an external supply of M2-like macrophages. Furthermore, IL-1α and osteopontin were suggested to be mediators of M2-like macrophage-induced fibroblast activation. In addition, IL-4 administration achieved a targeted increase in the number of M2-like macrophages and enhanced the post-MI prognosis of WT mice, corresponding with amplified fibroblast activation and formation of more supportive fibrous tissues in the infarcts. Together, these data demonstrate that M2-like macrophages critically determine the repair of infarcted adult murine heart by regulating fibroblast activation and suggest that IL-4 is a potential biological drug for treating MI.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

