Non-Invasive Ultrasound Liver Ablation Using Histotripsy: Chronic Study in an In Vivo Rodent Model
- PMID: 27140521
- PMCID: PMC4912895
- DOI: 10.1016/j.ultrasmedbio.2016.03.018
Non-Invasive Ultrasound Liver Ablation Using Histotripsy: Chronic Study in an In Vivo Rodent Model
Abstract
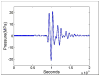
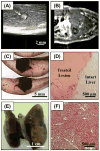
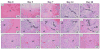
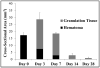
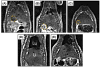
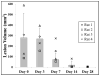
Hepatocellular carcinoma, or liver cancer, has the fastest growing incidence among cancers in the United States. Current liver ablation methods are thermal-based and share limitations due to the heat sink effect from the blood flow through the highly vascular liver. Recently, our group has investigated histotripsy as a non-invasive liver cancer ablation method. Histotripsy is a non-thermal ultrasonic ablation method that fractionates tissue through the control of acoustic cavitation. Previous experiments in an in vivo porcine model show that histotripsy can create well-confined lesions in the liver through ribcage obstruction without damaging the overlying ribs and other tissues. Histotripsy can also completely fractionate liver tissue surrounding major vessels while preserving the vessels. In this study, we investigate the long-term effects of histotripsy liver ablation in a rodent model. We hypothesize that the fractionated histotripsy lesion will be resorbed by the liver, resulting in effective tissue healing. To test this hypothesis, the livers of 20 healthy rats were treated with histotripsy using an 8-element 1-MHz histotripsy transducer. Rats were euthanized after 0, 3, 7, 14 and 28 days (n = 4). In vivo and post mortem results showed histotripsy lesions were successfully generated through the intact abdomen in all 20 rats. Magnetic resonance imaging found primarily negative contrast on day 0, positive contrast on day 3 and rapid normalization of signal intensity thereafter (i.e., signal amplitude returned to baseline levels seen in healthy liver tissue). Histologically, lesions were completely fractionated into an acellular homogenate. The lesions had a maximum cross-sectional area of 17.2 ± 1.9 mm(2) and sharp boundaries between the lesion and the healthy surrounding tissue after treatment. As the animals recovered after treatment, the histotripsy tissue homogenate was almost completely replaced by regenerated liver parenchyma, resulting in a small fibrous lesion (<1 mm(2) maximum cross-section) remaining after 28 d. The results of this study suggest that histotripsy has potential as a non-invasive liver ablation method for effective tissue removal.
Keywords: Cavitation; Histotripsy; Liver cancer; Non-invasive tissue ablation; Ultrasound.
Copyright © 2016 World Federation for Ultrasound in Medicine & Biology. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- ACS. Cancer Facts and Figures 2015. American Cancer Society; 2015.
-
- Allen SP, Hall TL, Cain CA, Hernandez-Garcia L. Controlling cavitation-based image contrast in focused ultrasound histotripsy surgery. Magn Reson Med. 2014 - PubMed
-
- Apfel RE, Holland CK. Gauging the likelihood of cavitation from short-pulse, low-duty cycle diagnostic ultrasound. Ultrasound Med Biol. 1991;17:179–85. - PubMed
-
- Aschoff AJ, Merkle EM, Wong V, Zhang Q, Mendez MM, Duerk JL, Lewin JS. How does alteration of hepatic blood flow affect liver perfusion and radiofrequency-induced thermal lesion size in rabbit liver? J Magn Reson Imaging. 2001;13:57–63. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

