PD-1 expression on dendritic cells suppresses CD8+ T cell function and antitumor immunity
- PMID: 27141339
- PMCID: PMC4839350
- DOI: 10.1080/2162402X.2015.1085146
PD-1 expression on dendritic cells suppresses CD8+ T cell function and antitumor immunity
Abstract
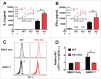
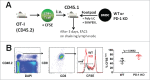
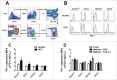
Programmed death one (PD-1) is a well-established co-inhibitory regulator that suppresses proliferation and cytokine production of T cells. Despite remarkable progress in delineating the functional roles of PD-1 on T lymphocytes, little is known about the regulatory role of PD-1 expressed on myeloid cells such as dendritic cells (DCs). Here, we show that CD8+ T cells can be more potently activated to secrete IL-2 and IFNγ by PD-1-deficient DCs compared to wild-type DCs. Adoptive transfer of PD-1-deficient DCs demonstrated their superior capabilities in inducing antigen-specific CD8+ T cell proliferation in vivo. In addition, we provide first evidence demonstrating the existence of peripheral blood DCs and CD11c+ tumor-infiltrating myeloid cells that co-express PD-1 in patients with hepatocellular carcinoma (HCC). The existence of PD-1-expressing HCC-infiltrating DCs (HIDCs) was further supported in a mouse model of HCC. Intratumoral transfer of PD-1-deficient DCs rendered recipient mice resistant to the growth of HCC by promoting tumor-infiltrating CD8+ effector T cells to secrete perforin and granzyme B. This novel finding provides a deeper understanding of the role of PD-1 in immune regulation and has significant implications for cancer immunotherapies targeting PD-1.
Keywords: Antitumor immunity; Programmed death one (PD-1); cancer immunotherapy; dendritic cell; hepatocellular carcinoma; liver cancer.
Figures


References
-
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008; 26:677-704; PMID:18173375; http://dx.doi.org/10.1146/annurev.immunol.26.021607.090331. - DOI - PMC - PubMed
-
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999; 11:141-51; PMID:10485649; http://dx.doi.org/10.1016/S1074-7613(00)80089-8. - DOI - PubMed
-
- Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001; 291:319-22; PMID:11209085; http://dx.doi.org/10.1126/science.291.5502.319. - DOI - PubMed
-
- Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T. Establishment of NOD-Pdcd1-/- mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci U S A 2005; 102:11823-8; PMID:16087865; http://dx.doi.org/10.1073/pnas.0505497102. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
