A novel regulation of PD-1 ligands on mesenchymal stromal cells through MMP-mediated proteolytic cleavage
- PMID: 27141350
- PMCID: PMC4839348
- DOI: 10.1080/2162402X.2015.1091146
A novel regulation of PD-1 ligands on mesenchymal stromal cells through MMP-mediated proteolytic cleavage
Abstract
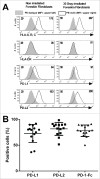
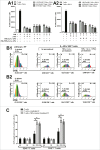
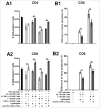
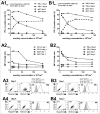
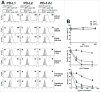
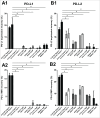
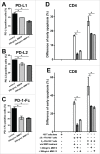
Whether fibroblasts regulate immune response is a crucial issue in the modulation of inflammatory responses. Herein, we demonstrate that foreskin fibroblasts (FFs) potently inhibit CD3+ T cell proliferation through a mechanism involving early apoptosis of activated T cells. Using blocking antibodies, we demonstrate that the inhibition of T cell proliferation occurs through cell-to-cell interactions implicating PD-1 receptor expressed on T cells and its ligands, PD-L1 and PD-L2, on fibroblasts. Dual PD-1 ligand neutralization is required to abrogate (i) binding of the PD-1-Fc fusion protein, (ii) early apoptosis of T cells, and (iii) inhibition of T cell proliferation. Of utmost importance, we provide the first evidence that PD-1 ligand expression is regulated through proteolytic cleavage by endogenous matrix metalloproteinases (MMPs) without transcriptional alteration during culture-time. Using (i) different purified enzymatic activities, (ii) MMP-specific inhibitors, and (iii) recombinant human MMP-9 and MMP-13, we demonstrated that in contrast to CD80/CD86, PD-L1 was selectively cleaved by MMP-13, while PD-L2 was sensitive to broader MMP activities. Their cleavage by exogenous MMP-9 and MMP-13 with loss of PD-1 binding domain resulted in the reversion of apoptotic signals on mitogen-activated CD3+ T cells. We suggest that MMP-dependent cleavage of PD-1 ligands on fibroblasts may limit their immunosuppressive capacity and thus contribute to the exacerbation of inflammation in tissues. In contrast, carcinoma-associated fibroblasts appear PD-1 ligand-depleted through MMP activity that may impair physical deletion of exhausted defective memory T cells through apoptosis and facilitate their regulatory functions. These observations should be considered when using the powerful PD-1/PD-L1 blocking immunotherapies.
Keywords: Apoptosis; MMP-13; PD-L1; PD-L2; PD1; fibroblasts; immunosuppression.
Figures




References
-
- Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 2013; 13:392-402; PMID:24094322; http://dx.doi.org/10.1016/j.stem.2013.09.006 - DOI - PubMed
-
- Frenette PS, Pinho S, Lucas D, Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol 2013; 31:285-316; PMID:23298209; http://dx.doi.org/10.1146/annurev-immunol-032712-095919 - DOI - PubMed
-
- Le BK, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol 2012; 12:383-96; PMID:22531326; http://dx.doi.org/10.1038/nri3209 - DOI - PubMed
-
- Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, Brown PO. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci U S A 2002; 99:12877-82; PMID:12297622; http://dx.doi.org/10.1073/pnas.162488599 - DOI - PMC - PubMed
-
- Haniffa MA, Wang XN, Holtick U, Rae M, Isaacs JD, Dickinson AM, Hilkens CM, Collin MP. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol 2007; 179:1595-604; PMID:17641026; http://dx.doi.org/10.4049/jimmunol.179.3.1595 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
