Characterization of the first-in-class T-cell-engaging bispecific single-chain antibody for targeted immunotherapy of solid tumors expressing the oncofetal protein claudin 6
- PMID: 27141353
- PMCID: PMC4839326
- DOI: 10.1080/2162402X.2015.1091555
Characterization of the first-in-class T-cell-engaging bispecific single-chain antibody for targeted immunotherapy of solid tumors expressing the oncofetal protein claudin 6
Abstract
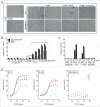
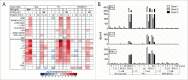
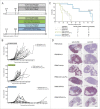
The fetal tight junction molecule claudin 6 (CLDN6) is virtually absent from any normal tissue, whereas it is aberrantly and frequently expressed in various cancers of high medical need. We engineered 6PHU3, a T-cell-engaging bispecific single chain molecule (bi-(scFv)2) with anti-CD3/anti-CLDN6 specificities, and characterized its pharmacodynamic properties. Our data show that upon engagement by 6PHU3, T cells strongly upregulate cytotoxicity and activation markers, proliferate and acquire an effector phenotype. 6PHU3 exerts potent killing of cancer cells in vitro with EC50 values in the pg/mL range. Subcutaneous xenograft tumors in NSG mice engrafted with human PBMCs are eradicated by 6PHU3 treatment and survival of mice is significantly prolonged. Tumors of 6PHU3-treated mice are strongly infiltrated with activated CD4+, CD8+ T cells and TEM type cells but not Tregs and display a general activation of a mostly inflammatory phenotype. These effects are only observed upon bispecific but not monospecific engagement of 6PHU3. Together with the exceptionally cancer cell selective expression of the oncofetal tumor marker CLDN6, this provides a safeguard with regard to toxicity. In summary, our data shows that the concept of T-cell redirection combined with that of highly selective targeting of CLDN6-positive solid tumors is effective. Thus, exploring 6PHU3 for clinical therapy is warranted.
Keywords: Bispecific antibody; T cell engagement; T-cell engager; ideal target; oncofetal tumor marker; solid tumors; targeted immunotherapy; tumor-infiltrating lymphocytes; xenograft mouse model.
Figures


References
-
- Clark MR, Waldmann H. T-cell killing of target cells induced by hybrid antibodies: comparison of two bispecific monoclonal antibodies. J Natl Cancer Inst 1987; 79:1393-401; PMID:3121901 - PubMed
-
- Lanzavecchia A, Scheidegger D. The use of hybrid hybridomas to target human cytotoxic T lymphocytes. Eur J Immunol 1987; 17:105-11; PMID:3102250; http://dx.doi.org/10.1002/eji.1830170118 - DOI - PubMed
-
- Sahin U, Hartmann F, Senter P, Pohl C, Engert A, Diehl V, Pfreundschuh M. Specific activation of the prodrug mitomycin phosphate by a bispecific anti-CD30/anti-alkaline phosphatase monoclonal antibody. Cancer Res 1990; 50:6944-48; PMID:2170012 - PubMed
-
- Staerz UD, Kanagawa O, Bevan MJ. Hybrid antibodies can target sites for attack by T cells. Nature 1985; 314:628-31; PMID:2859527; http://dx.doi.org/10.1038/314628a0 - DOI - PubMed
-
- Ruf P, Gires O, Jäger M, Fellinger K, Atz J, Lindhofer H. Characterisation of the new EpCAM-specific antibody HO-3: implications for trifunctional antibody immunotherapy of cancer. Br J Cancer 2007; 97:315-21; PMID:17622246; http://dx.doi.org/10.1038/sj.bjc.6603881 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
