Antitumor cytotoxicity induced by bone-marrow-derived antigen-presenting cells is facilitated by the tumor suppressor protein p53 via regulation of IL-12
- PMID: 27141366
- PMCID: PMC4839352
- DOI: 10.1080/2162402X.2015.1112941
Antitumor cytotoxicity induced by bone-marrow-derived antigen-presenting cells is facilitated by the tumor suppressor protein p53 via regulation of IL-12
Abstract
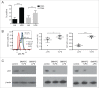
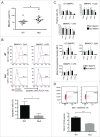
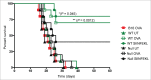
Activated antigen-presenting cells (APC) deliver the three signals cytotoxic T cells require to differentiate into effector cells that destroy the tumor. These comprise antigen, co-stimulatory signals and cytokines. Once these cells have carried out their function, they apoptose. We hypothesized that the tumor suppressor protein, p53, played an important role in generating the antitumor response facilitated by APC. CD11c+ APC derived from p53 wild-type (wt) mouse (wt p53) GM-CSF bone marrow cultures (BMAPC) and activated had reduced survival compared to BMAPC from p53 null consistent with p53-mediated apoptosis following activation. There was a lower percentage of antigenic peptide/MHC I complexes on antigen-pulsed p53 null cells suggesting p53 played a role in antigen processing but there was no difference in antigen-specific T cell proliferative responses to these cells in vivo. In contrast, antigen-specific cytotoxicity in vivo was markedly reduced in response to p53 null BMAPC. When these cells were pulsed with a model tumor antigen and delivered as a prophylactic vaccination, they provided no protection against melanoma cell growth whereas wt BMAPC were very effective. This suggested that p53 might regulate the requisite third signal and, indeed, we found that p53 null BMAPC produced less IL-12 than wt p53 BMAPC and that p53 bound to the promoter region of IL-12. This work suggests that p53 in activated BMAPC is associated with the generation of IL-12 required for the differentiation of cytotoxic immune responses and an effective antitumor response. This is a completely new role for this protein that has implications for BMAPC-mediated immunotherapy.
Keywords: Cytotoxic immune response; dendritic cells; interleukin 12; p53 tumor suppressor protein; tumor vaccination.
Figures



Similar articles
-
Priming to mycobacterial antigen in vivo using antigen-pulsed antigen presenting cells generated in vitro is influenced by the dose and presence of IL-4 in APC cultures.Scand J Immunol. 1997 Jul;46(1):1-9. doi: 10.1046/j.1365-3083.1997.d01-88.x. Scand J Immunol. 1997. PMID: 9246202
-
Human monocyte-derived dendritic cells pulsed with wild-type p53 protein efficiently induce CTLs against p53 overexpressing human cancer cells.Clin Cancer Res. 2005 Feb 1;11(3):1312-8. Clin Cancer Res. 2005. PMID: 15709203
-
Antigen presentation and immune regulatory capacity of immature and mature-enriched antigen presenting (dendritic) cells derived from human bone marrow.Hum Immunol. 2004 Feb;65(2):93-103. doi: 10.1016/j.humimm.2003.11.002. Hum Immunol. 2004. PMID: 14969764
-
Clinically feasible approaches to potentiating cancer cell-based immunotherapies.Hum Vaccin Immunother. 2015;11(4):851-69. doi: 10.1080/21645515.2015.1009814. Hum Vaccin Immunother. 2015. PMID: 25933181 Free PMC article. Review.
-
Therapeutic gene modified cell based cancer vaccines.Gene. 2013 Aug 10;525(2):200-7. doi: 10.1016/j.gene.2013.03.056. Epub 2013 Apr 6. Gene. 2013. PMID: 23566846 Review.
Cited by
-
p53 suppresses MHC class II presentation by intestinal epithelium to protect against radiation-induced gastrointestinal syndrome.Nat Commun. 2024 Jan 2;15(1):137. doi: 10.1038/s41467-023-44390-w. Nat Commun. 2024. PMID: 38167344 Free PMC article.
-
∆133p53 isoform promotes tumour invasion and metastasis via interleukin-6 activation of JAK-STAT and RhoA-ROCK signalling.Nat Commun. 2018 Jan 17;9(1):254. doi: 10.1038/s41467-017-02408-0. Nat Commun. 2018. PMID: 29343721 Free PMC article.
-
MDM2 inhibitors in cancer immunotherapy: Current status and perspective.Genes Dis. 2024 Mar 28;11(6):101279. doi: 10.1016/j.gendis.2024.101279. eCollection 2024 Nov. Genes Dis. 2024. PMID: 39263534 Free PMC article. Review.
-
Activation of p53 in Immature Myeloid Precursor Cells Controls Differentiation into Ly6c+CD103+ Monocytic Antigen-Presenting Cells in Tumors.Immunity. 2018 Jan 16;48(1):91-106.e6. doi: 10.1016/j.immuni.2017.12.014. Immunity. 2018. PMID: 29343444 Free PMC article.
-
Inverse correlation between TP53 gene status and PD-L1 protein levels in a melanoma cell model depends on an IRF1/SOX10 regulatory axis.Cell Mol Biol Lett. 2024 Sep 5;29(1):117. doi: 10.1186/s11658-024-00637-y. Cell Mol Biol Lett. 2024. PMID: 39237877 Free PMC article.
References
-
- Kushwah R, Hu J. Dendritic cell apoptosis: regulation of tolerance vs. immunity. J Immunol 2010; 185:795-802; PMID:20601611; http://dx.doi.org/2063680510.4049/jimmunol.1000325. - DOI - PubMed
-
- Chen M, Wang J. Programmed cell death of dendritic cells in immune regulation. Immunol Rev 2010; 236:11-27; PMID:20636805; http://dx.doi.org/10.1111/j.1600-065X.2010.00916.x. - DOI - PMC - PubMed
-
- Oren M. Decision making by p53: life, death and cancer. Cell Death Diff 2003; 10:431-42; PMID:12719720; http://dx.doi.org/10.1038/sj.cdd.4401183. - DOI - PubMed
-
- Braithwaite AW, Royds JA, Jackson P. The p53 story: layers of complexity. Carcinogenesis 2005; 26:1161-9; PMID:15817608; http://dx.doi.org/10.1093/carcin/bgi091. - DOI - PubMed
-
- Lowe SW, Jacks T, Housman DE, Ruley HE. Abrogation of oncogene-associated apoptosis allows transformation of p53-deficient cells. Proc Natl Acad Sci U S A 1994; 91:2026-30; PMID:8134344; http://dx.doi.org/10.1073/pnas.91.6.2026. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
