Consensus Statement on Electronic Health Predictive Analytics: A Guiding Framework to Address Challenges
- PMID: 27141516
- PMCID: PMC4837887
- DOI: 10.13063/2327-9214.1163
Consensus Statement on Electronic Health Predictive Analytics: A Guiding Framework to Address Challenges
Abstract
Context: The recent explosion in available electronic health record (EHR) data is motivating a rapid expansion of electronic health care predictive analytic (e-HPA) applications, defined as the use of electronic algorithms that forecast clinical events in real time with the intent to improve patient outcomes and reduce costs. There is an urgent need for a systematic framework to guide the development and application of e-HPA to ensure that the field develops in a scientifically sound, ethical, and efficient manner.
Objectives: Building upon earlier frameworks of model development and utilization, we identify the emerging opportunities and challenges of e-HPA, propose a framework that enables us to realize these opportunities, address these challenges, and motivate e-HPA stakeholders to both adopt and continuously refine the framework as the applications of e-HPA emerge.
Methods: To achieve these objectives, 17 experts with diverse expertise including methodology, ethics, legal, regulation, and health care delivery systems were assembled to identify emerging opportunities and challenges of e-HPA and to propose a framework to guide the development and application of e-HPA.
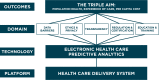
Findings: The framework proposed by the panel includes three key domains where e-HPA differs qualitatively from earlier generations of models and algorithms (Data Barriers, Transparency, and ETHICS) and areas where current frameworks are insufficient to address the emerging opportunities and challenges of e-HPA (Regulation and Certification; and Education and Training). The following list of recommendations summarizes the key points of the framework: Data Barriers: Establish mechanisms within the scientific community to support data sharing for predictive model development and testing.Transparency: Set standards around e-HPA validation based on principles of scientific transparency and reproducibility.
Ethics: Develop both individual-centered and society-centered risk-benefit approaches to evaluate e-HPA.Regulation and Certification: Construct a self-regulation and certification framework within e-HPA.Education and Training: Make significant changes to medical, nursing, and paraprofessional curricula by including training for understanding, evaluating, and utilizing predictive models.
Keywords: Clinical decision support systems; Ethics; Health Information Technology; Informatics; big data; electronic predictive analytics; predictive models.
Figures
References
-
- Knaus WA, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29. - PubMed
-
- Caro JJ, Briggs AH, Siebert U, et al. Modeling good research practices - overview: A report of the ISPOR-SMDM modeling good research practices task force-1. Value Health. 2012;15:796–803. - PubMed
-
- Eddy DM, Hollingworth W, Caro JJ, et al. Model transparency and validation: A report of the ISPOR-SMDM modeling good research practices task force-4. Value Health. 2012;15:843–50. - PubMed
-
- Briggs AH, Weinstein MC, Fenwick E, et al. Model parameter estimation and uncertainty analysis: A report of the ISPOR-SMDM modeling good research practices task force-6. Value Health. 2012;15:835–42. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
