Extracting Electronic Health Record Data in a Practice-Based Research Network: Processes to Support Translational Research across Diverse Practice Organizations
- PMID: 27141519
- PMCID: PMC4827782
- DOI: 10.13063/2327-9214.1206
Extracting Electronic Health Record Data in a Practice-Based Research Network: Processes to Support Translational Research across Diverse Practice Organizations
Abstract
Context: The widespread adoption of electronic health records (EHRs) offers significant opportunities to conduct research with clinical data from patients outside traditional academic research settings. Because EHRs are designed primarily for clinical care and billing, significant challenges are inherent in the use of EHR data for clinical and translational research. Efficient processes are needed for translational researchers to overcome these challenges. The Data QUEST Coordinating Center (DQCC), which oversees Data Query Extraction Standardization Translation (Data QUEST) - a primary-care, EHR data-sharing infrastructure - created processes that guide EHR data extraction for clinical and translational research across these diverse practices. We describe these processes and their application in a case example.
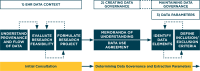
Case description: The DQCC process for developing EHR data extractions not only supports researchers' access to EHR data, but supports this access for the purpose of answering scientific questions. This process requires complex coordination across multiple domains, including the following: (1) understanding the context of EHR data; (2) creating and maintaining a governance structure to support exchange of EHR data; and (3) defining data parameters that are used in order to extract data from the EHR. We use the Northwest-Alaska Pharmacogenomics Research Network (NWA-PGRN) as a case example that focuses on pharmacogenomic discovery and clinical applications to describe the DQCC process. The NWA-PGRN collaborates with Data QUEST to explore ways to leverage primary-care EHR data to support pharmacogenomics research.
Findings: Preliminary analysis on the case example shows that initial decisions about how researchers define the study population can influence study outcomes.
Major themes and conclusions: The experience of the DQCC demonstrates that coordinating centers provide expertise in helping researchers understand the context of EHR data, create and maintain governance structures, and guide the definition of parameters for data extractions. This expertise is critical to supporting research with EHR data. Replication of these strategies through coordinating centers may lead to more efficient translational research. Investigators must also consider the impact of initial decisions in defining study groups that may potentially affect outcomes.
Keywords: electronic health records; governance; primary care.
Figures
Similar articles
-
Electronic Health Record Challenges, Workarounds, and Solutions Observed in Practices Integrating Behavioral Health and Primary Care.J Am Board Fam Med. 2015 Sep-Oct;28 Suppl 1(Suppl 1):S63-72. doi: 10.3122/jabfm.2015.S1.150133. J Am Board Fam Med. 2015. PMID: 26359473 Free PMC article.
-
The Effectiveness of Integrated Care Pathways for Adults and Children in Health Care Settings: A Systematic Review.JBI Libr Syst Rev. 2009;7(3):80-129. doi: 10.11124/01938924-200907030-00001. JBI Libr Syst Rev. 2009. PMID: 27820426
-
Developing Governance for Federated Community-based EHR Data Sharing.AMIA Jt Summits Transl Sci Proc. 2014 Apr 7;2014:71-6. eCollection 2014. AMIA Jt Summits Transl Sci Proc. 2014. PMID: 25717404 Free PMC article.
-
Facilitating biomedical researchers' interrogation of electronic health record data: Ideas from outside of biomedical informatics.J Biomed Inform. 2016 Apr;60:376-84. doi: 10.1016/j.jbi.2016.03.004. Epub 2016 Mar 10. J Biomed Inform. 2016. PMID: 26972838 Free PMC article. Review.
-
Big data from electronic health records for early and late translational cardiovascular research: challenges and potential.Eur Heart J. 2018 Apr 21;39(16):1481-1495. doi: 10.1093/eurheartj/ehx487. Eur Heart J. 2018. PMID: 29370377 Free PMC article. Review.
Cited by
-
Responsible data sharing in a big data-driven translational research platform: lessons learned.BMC Med Inform Decis Mak. 2019 Dec 30;19(1):283. doi: 10.1186/s12911-019-1001-y. BMC Med Inform Decis Mak. 2019. PMID: 31888593 Free PMC article.
-
Exploring completeness in clinical data research networks with DQe-c.J Am Med Inform Assoc. 2018 Jan 1;25(1):17-24. doi: 10.1093/jamia/ocx109. J Am Med Inform Assoc. 2018. PMID: 29069394 Free PMC article.
-
Defining Major Depressive Disorder Cohorts Using the EHR: Multiple Phenotypes Based on ICD-9 Codes and Medication Orders.Neurol Psychiatry Brain Res. 2020 Jun;36:18-26. doi: 10.1016/j.npbr.2020.02.002. Epub 2020 Feb 21. Neurol Psychiatry Brain Res. 2020. PMID: 32218644 Free PMC article.
-
Service utilization and chronic condition outcomes among primary care patients with substance use disorders and co-occurring chronic conditions.J Subst Abuse Treat. 2020 Mar;112S:49-55. doi: 10.1016/j.jsat.2020.02.008. J Subst Abuse Treat. 2020. PMID: 32220411 Free PMC article.
-
Building a practice-based research network for healthcare integration: a protocol paper for a mixed-method project.BMJ Open. 2022 Jun 9;12(6):e060524. doi: 10.1136/bmjopen-2021-060524. BMJ Open. 2022. PMID: 35680253 Free PMC article.
References
-
- Jensen PB, Jensen LJ, Brunak S. Mining electronic health records: towards better research applications and clinical care. Nat Rev Genet. 2012 May 2;13(6):395–405. - PubMed
-
- Prokosch HU, Ganslandt T. Perspectives for medical informatics. Reusing the electronic medical record for clinical research. Methods Inf Med. 2009;48(1):38–44. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources