Multiscale Poly-(ϵ-caprolactone) Scaffold Mimicking Nonlinearity in Tendon Tissue Mechanics
- PMID: 27141530
- PMCID: PMC4851111
- DOI: 10.1007/s40883-016-0008-5
Multiscale Poly-(ϵ-caprolactone) Scaffold Mimicking Nonlinearity in Tendon Tissue Mechanics
Abstract
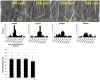
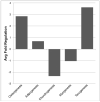
Regenerative medicine plays a critical role in the future of medicine. However, challenges remain to balance stem cells, biomaterial scaffolds, and biochemical factors to create successful and effective scaffold designs. This project analyzes scaffold architecture with respect to mechanical capability and preliminary mesenchymal stem cell response for tendon regeneration. An electrospun fiber scaffold with tailorable properties based on a "Chinese-fingertrap" design is presented. The unique criss-crossed fiber structures demonstrate non-linear mechanical response similar to that observed in native tendon. Mechanical testing revealed that optimizing the fiber orientation resulted in the characteristic "S"-shaped curve, demonstrating a toe region and linear elastic region. This project has promising research potential across various disciplines: vascular engineering, nerve regeneration, and ligament and tendon tissue engineering.
Keywords: bioinstructive scaffold; electrospinning; poly-(ϵ-caprolactone); regenerative medicine; tendon.
Figures



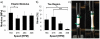

References
-
- Maffulli N, Renstrom P, Leadbetter WB, editors. Tendon Injuries: Basic Science and Clinical Medicine. Springer; 2005.
-
- Chen J, Xu J, Wang A, Zheng M. Scaffolds for Tendon and Ligament Repair: Review of the Efficacy of Commercial Products. Expert Rev Med Devices. 2009;6:61–73. doi:10.1586/17434440.6.1.61. - PubMed
-
- Martin B, Burr DB, Sharkey NA. Mechanical Properties of Ligament and Tendon. In: Martin B, Burr DB, Sharkey NA, editors. Skelet. Tissue Mech. Springer; New York, NY: 1998. pp. 309–47.
-
- Lim JJ, Temenoff JS. Tendon and Ligament Tissue Engineering: Restoring Tendon/Ligament and Its Interfaces. In: Meyer U, Meyer T, Handschel J, Wiesmann HP, editors. Fundam. Tissue Eng. Regen. Med. Springer; 2009. pp. 255–69. doi:10.1007/978-3-540-77755-7.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
