Molecular identification and antigenic characterization of a merozoite surface antigen and a secreted antigen of Babesia canis (BcMSA1 and BcSA1)
- PMID: 27141812
- PMCID: PMC4855366
- DOI: 10.1186/s13071-016-1518-1
Molecular identification and antigenic characterization of a merozoite surface antigen and a secreted antigen of Babesia canis (BcMSA1 and BcSA1)
Abstract
Background: Babesia canis is an apicomplexan tick-transmitted hemoprotozoan responsible for causing canine babesiosis in Europe and west Asia. Despite its importance, there is no known rapid diagnostic kit detection of B. canis infection in dogs. The present study identified two novel antigens of B. canis and used the recombinant antigens to establish a rapid, specific and sensitive serodiagnostic technique for detection of B. canis infection.
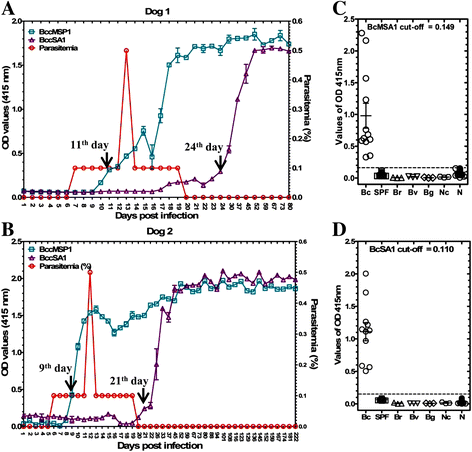
Methods: A complementary DNA (cDNA) expression library was constructed from the mRNA of B. canis and immunoscreened using B. canis-infected dog sera. The cDNAs encoding a merozoite surface antigen and a secreted antigen protein were identified and designated as BcMSA1 and BcSA1, respectively. The recombinant BcMSA1 and BcSA1 (rBcMSA1 and rBcSA1) expressed in Escherichia coli were purified and injected into mice for production of anti-sera. The native proteins were characterized by Western blot analysis and immunofluorescence. Furthermore, indirect enzyme-linked immunosorbent assays (iELISA) and rapid immunochromatographic tests (ICT) based on rBcMSA1 or rBcSA1 were established and evaluated to test specific antibodies in consecutive plasma samples from two B. canis-infected dogs.
Results: Antiserum raised against rBcMSA1 and rBcSA1 recognized the 39 kDa and 44 kDa native proteins by Western blot analysis, respectively. In addition, immunofluorescence and confocal microscopic observations revealed that BcMSA1 was found on the surface of parasites. However, BcSA1 localized in the matrix of the merozoites. The ELISA and ICT based on rBcMSA1 or rBcSA1 could detect specific antibodies in consecutive plasma samples from two B. canis-infected dogs. They showed no cross-reactions against the serum samples collected from dogs experimentally infected with closely related parasites.
Conclusion: Taken together, the current results indicated that the rBcMSA1 and rBcSA1 are promising serodiagnostic antigens for developing iELISA and ICT to detect B. canis infection. To our knowledge, this study is the first to report BcMSA1 and BcSA1 as potential antigenic proteins for serodiagnosis of B. canis infection in dogs.
Keywords: Babesia canis; BcMSA1; BcSA1; Canine babesiosis; ELISA; Immunochromatographic tests.
Figures




Similar articles
-
A novel 57-kDa merozoite protein of Babesia gibsoni is a prospective antigen for diagnosis and serosurvey of canine babesiosis by enzyme-linked immunosorbent assay.Vet Parasitol. 2007 Oct 21;149(1-2):85-94. doi: 10.1016/j.vetpar.2007.06.025. Epub 2007 Aug 15. Vet Parasitol. 2007. PMID: 17706873
-
Molecular characterization and antigenic properties of a novel Babesia gibsoni glutamic acid-rich protein (BgGARP).Exp Parasitol. 2013 Oct;135(2):414-20. doi: 10.1016/j.exppara.2013.08.005. Epub 2013 Aug 19. Exp Parasitol. 2013. PMID: 23968686
-
Identification and expression of a 50-kilodalton surface antigen of Babesia gibsoni and evaluation of its diagnostic potential in an enzyme-linked immunosorbent assay.J Clin Microbiol. 2001 Jul;39(7):2603-9. doi: 10.1128/JCM.39.7.2603-2609.2001. J Clin Microbiol. 2001. PMID: 11427577 Free PMC article.
-
Genetic basis for GPI-anchor merozoite surface antigen polymorphism of Babesia and resulting antigenic diversity.Vet Parasitol. 2006 May 31;138(1-2):33-49. doi: 10.1016/j.vetpar.2006.01.038. Epub 2006 Mar 23. Vet Parasitol. 2006. PMID: 16551492 Review.
-
Vaccination against babesiosis using recombinant GPI-anchored proteins.Int J Parasitol. 2019 Feb;49(2):175-181. doi: 10.1016/j.ijpara.2018.12.002. Epub 2019 Jan 24. Int J Parasitol. 2019. PMID: 30684517 Review.
Cited by
-
Major Surface Antigens in Zoonotic Babesia.Pathogens. 2022 Jan 15;11(1):99. doi: 10.3390/pathogens11010099. Pathogens. 2022. PMID: 35056047 Free PMC article. Review.
-
Immune Response of Mice Against Babesia canis Antigens is Enhanced When Antigen is Coupled to Gold Nanoparticles.Acta Parasitol. 2021 Jun;66(2):493-500. doi: 10.1007/s11686-020-00305-z. Epub 2020 Nov 9. Acta Parasitol. 2021. PMID: 33165701
-
Genome-wide analysis of gene expression and protein secretion of Babesia canis during virulent infection identifies potential pathogenicity factors.Sci Rep. 2017 Jun 13;7(1):3357. doi: 10.1038/s41598-017-03445-x. Sci Rep. 2017. PMID: 28611446 Free PMC article.
-
Applying Machine Learning to Predict the Exportome of Bovine and Canine Babesia Species That Cause Babesiosis.Pathogens. 2021 May 27;10(6):660. doi: 10.3390/pathogens10060660. Pathogens. 2021. PMID: 34071992 Free PMC article.
-
Knowledge, Attitudes, and Practices of Pastoralists Towards Tick Bites, and Tick Control in Plateau State, Nigeria.Acta Parasitol. 2023 Jun;68(2):372-384. doi: 10.1007/s11686-023-00670-5. Epub 2023 Mar 28. Acta Parasitol. 2023. PMID: 36976439
References
-
- Carret C, Walas F, Carcy B, Grande N, Précigout E, Moubri K, Schetters TP, Gorenflot A. Babesia canis canis, Babesia canis vogeli, Babesia canis rossi: differentiation of the three subspecies by a restriction fragment length polymorphism analysis on amplified small subunit ribosomal RNA genes. J Eukaryot Microbiol. 1999;46(3):298–303. doi: 10.1111/j.1550-7408.1999.tb05128.x. - DOI - PubMed
-
- Kubelová M, Sedlák K, Panev A, Siroký P. Conflicting results of serological, PCR and microscopic methods clarify the various risk levels of canine babesiosis in Slovakia: A complex approach to Babesia canis diagnostics. Vet Parasitol. 2013;191(3–4):353–7. doi: 10.1016/j.vetpar.2012.09.016. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

