Co-culture with neonatal cardiomyocytes enhances the proliferation of iPSC-derived cardiomyocytes via FAK/JNK signaling
- PMID: 27141946
- PMCID: PMC4855360
- DOI: 10.1186/s12861-016-0112-2
Co-culture with neonatal cardiomyocytes enhances the proliferation of iPSC-derived cardiomyocytes via FAK/JNK signaling
Abstract
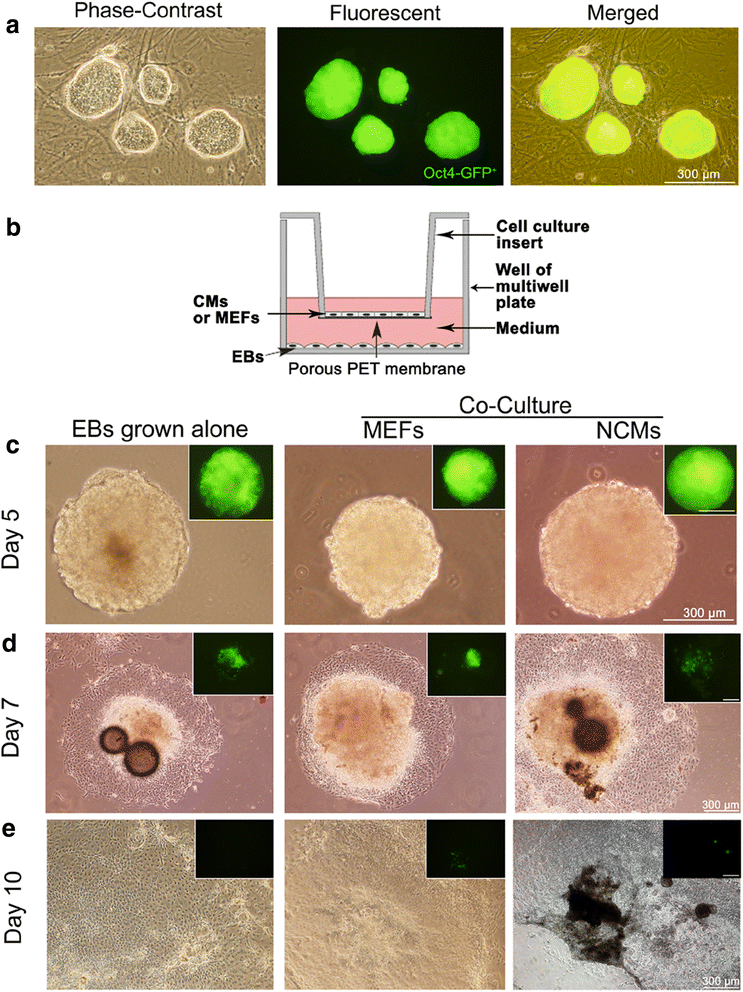
Background: We previously reported that the pluripotent stem cells can differentiate into cardiomyocytes (CMs) by co-culture with neonatal CMs (NCMs) in vitro. However, the involving mechanism is not clear.
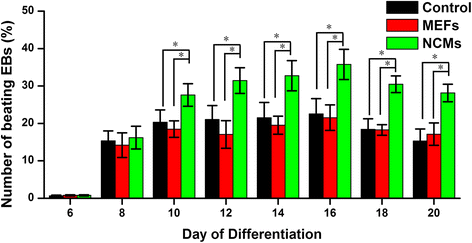
Methods: Mouse induced pluripotent stem cells (iPSCs) were cultured in hanging drops to form embryoid bodies (EBs) and to induce myocardial differentiation. Co-culture of EBs and NCMs was established in a transwell insert system, while EBs grown alone in the wells were used as controls.
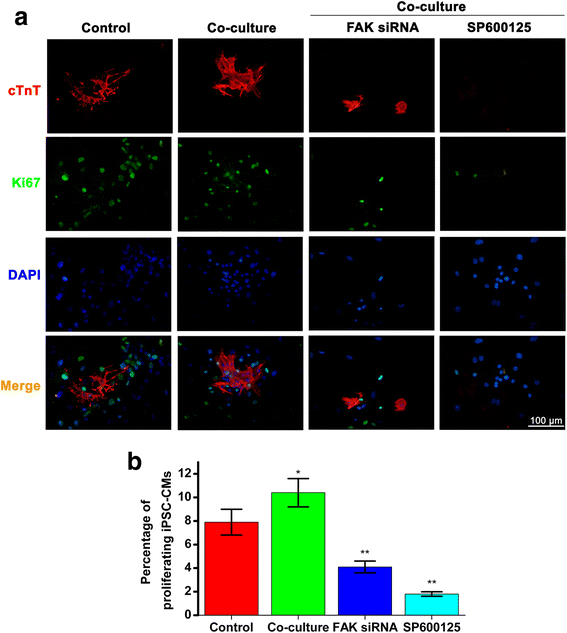
Results: Co-culture with NCMs markedly increased the generation of functional CMs from iPSCs. The focal adhesion kinase (FAK) phosphorylation, and c-Jun N-terminal kinase (JNK) phosphorylation in co-culture were higher than that in EBs grown alone. Treating FAK small interfering RNA (FAK siRNA) or specific inhibitor for JNK (SP600125) to iPSCs significantly reduced the phosphorylation of JNK and the expressions of Mef2c and Bcl-2. The expressions of cTnT and MLC-2V were also decreased. Our results revealed that co-culture with NCMs significantly enhance the differentiation ability of iPSCs by increasing Mef2c and Bcl-2 expressions concomitantly with a marked augment on cell proliferation through JNK signaling pathways.
Conclusions: These findings indicated that co-culture of EBs with NCMs induces genes expressed in a mature pattern and stimulates the proliferation of iPSC-derived CMs (iPS-CMs) by activating FAK/JNK signaling.
Keywords: Cardiomyocytes; Co-culture; FAK/JNK; Induced pluripotent stem cells; Proliferation.
Figures




Similar articles
-
The long-term differentiation of embryonic stem cells into cardiomyocytes: an indirect co-culture model.PLoS One. 2013;8(1):e55233. doi: 10.1371/journal.pone.0055233. Epub 2013 Jan 28. PLoS One. 2013. PMID: 23383121 Free PMC article.
-
Insulin-like growth factor binding protein 4 enhances cardiomyocytes induction in murine-induced pluripotent stem cells.J Cell Biochem. 2014 Sep;115(9):1495-504. doi: 10.1002/jcb.24804. J Cell Biochem. 2014. PMID: 24610529
-
A novel purification method of murine embryonic stem cell- and human-induced pluripotent stem cell-derived cardiomyocytes by simple manual dissociation.J Biomol Screen. 2012 Jun;17(5):683-91. doi: 10.1177/1087057111434145. Epub 2012 Jan 24. J Biomol Screen. 2012. PMID: 22274911
-
Induced pluripotent stem cell derived cardiac models: effects of Thymosin β4.Expert Opin Biol Ther. 2018 Jul;18(sup1):111-120. doi: 10.1080/14712598.2018.1473370. Expert Opin Biol Ther. 2018. PMID: 30063852 Review.
-
Progress in preclinical research on induced pluripotent stem cell therapy for acute myocardial infarction.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024 Apr 25;53(2):244-253. doi: 10.3724/zdxbyxb-2023-0402. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38594961 Free PMC article. Review. Chinese, English.
Cited by
-
Coordinated Proliferation and Differentiation of Human-Induced Pluripotent Stem Cell-Derived Cardiac Progenitor Cells Depend on Bone Morphogenetic Protein Signaling Regulation by GREMLIN 2.Stem Cells Dev. 2017 May 1;26(9):678-693. doi: 10.1089/scd.2016.0226. Epub 2017 Mar 20. Stem Cells Dev. 2017. PMID: 28125926 Free PMC article.
-
A New Method for Chromosomes Preparation by ATP-Competitive Inhibitor SP600125 via Enhancement of Endomitosis in Fish.Front Bioeng Biotechnol. 2021 Jan 13;8:606496. doi: 10.3389/fbioe.2020.606496. eCollection 2020. Front Bioeng Biotechnol. 2021. PMID: 33520960 Free PMC article.
-
FAK in Cancer: From Mechanisms to Therapeutic Strategies.Int J Mol Sci. 2022 Feb 2;23(3):1726. doi: 10.3390/ijms23031726. Int J Mol Sci. 2022. PMID: 35163650 Free PMC article. Review.
-
Human iPSC-derived cardiomyocytes and tissue engineering strategies for disease modeling and drug screening.Biotechnol Adv. 2017 Jan-Feb;35(1):77-94. doi: 10.1016/j.biotechadv.2016.12.002. Epub 2016 Dec 20. Biotechnol Adv. 2017. PMID: 28007615 Free PMC article. Review.
-
Co-culture of induced pluripotent stem cells with cardiomyocytes is sufficient to promote their differentiation into cardiomyocytes.PLoS One. 2020 Apr 3;15(4):e0230966. doi: 10.1371/journal.pone.0230966. eCollection 2020. PLoS One. 2020. PMID: 32243463 Free PMC article.
References
-
- Ma J, Guo L, Fiene SJ, Anson BD, Thomson JA, Kamp TJ, Kolaja KL, Swanson BJ, January CT. High purity human-induced pluripotent stem cell-derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. Am J Physiol Heart Circ Physiol. 2011;301(5):H2006–17. doi: 10.1152/ajpheart.00694.2011. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

