A ketogenic diet increases transport and oxidation of ketone bodies in RG2 and 9L gliomas without affecting tumor growth
- PMID: 27142056
- PMCID: PMC4933488
- DOI: 10.1093/neuonc/now088
A ketogenic diet increases transport and oxidation of ketone bodies in RG2 and 9L gliomas without affecting tumor growth
Abstract
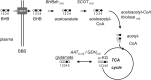
Background: The dependence of tumor cells, particularly those originating in the brain, on glucose is the target of the ketogenic diet, which creates a plasma nutrient profile similar to fasting: increased levels of ketone bodies and reduced plasma glucose concentrations. The use of ketogenic diets has been of particular interest for therapy in brain tumors, which reportedly lack the ability to oxidize ketone bodies and therefore would be starved during ketosis. Because studies assessing the tumors' ability to oxidize ketone bodies are lacking, we investigated in vivo the extent of ketone body oxidation in 2 rodent glioma models.
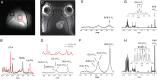
Methods: Ketone body oxidation was studied using (13)C MR spectroscopy in combination with infusion of a (13)C-labeled ketone body (beta-hydroxybutyrate) in RG2 and 9L glioma models. The level of ketone body oxidation was compared with nontumorous cortical brain tissue.
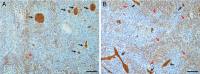
Results: The level of (13)C-beta-hydroxybutyrate oxidation in 2 rat glioma models was similar to that of contralateral brain. In addition, when glioma-bearing animals were fed a ketogenic diet, the ketone body monocarboxylate transporter was upregulated, facilitating uptake and oxidation of ketone bodies in the gliomas.
Conclusions: These results demonstrate that rat gliomas can oxidize ketone bodies and indicate upregulation of ketone body transport when fed a ketogenic diet. Our findings contradict the hypothesis that brain tumors are metabolically inflexible and show the need for additional research on the use of ketogenic diets as therapy targeting brain tumor metabolism.
Keywords: 13C MRS; MCT1; glioma; ketogenic diet; metabolism.
© The Author(s) 2016. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



Comment in
-
To diet or not to diet - that is still the question.Neuro Oncol. 2016 Aug;18(8):1035-6. doi: 10.1093/neuonc/now131. Neuro Oncol. 2016. PMID: 27382118 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

