Self-assembly of MPG1, a hydrophobin protein from the rice blast fungus that forms functional amyloid coatings, occurs by a surface-driven mechanism
- PMID: 27142249
- PMCID: PMC4855151
- DOI: 10.1038/srep25288
Self-assembly of MPG1, a hydrophobin protein from the rice blast fungus that forms functional amyloid coatings, occurs by a surface-driven mechanism
Abstract
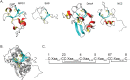
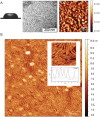
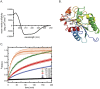
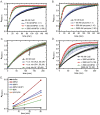
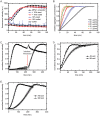
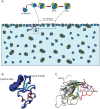
Rice blast is a devastating disease of rice caused by the fungus Magnaporthe oryzae and can result in loss of a third of the annual global rice harvest. Two hydrophobin proteins, MPG1 and MHP1, are highly expressed during rice blast infections. These hydrophobins have been suggested to facilitate fungal spore adhesion and to direct the action of the enzyme cutinase 2, resulting in penetration of the plant host. Therefore a mechanistic understanding of the self-assembly properties of these hydrophobins and their interaction with cutinase 2 is crucial for the development of novel antifungals. Here we report details of a study of the structure, assembly and interactions of these proteins. We demonstrate that, in vitro, MPG1 assembles spontaneously into amyloid structures while MHP1 forms a non-fibrillar film. The assembly of MPG1 only occurs at a hydrophobic:hydrophilic interface and can be modulated by MHP1 and other factors. We further show that MPG1 assemblies can much more effectively retain cutinase 2 activity on a surface after co-incubation and extensive washing compared with other protein coatings. The assembly and interactions of MPG1 and MHP1 at hydrophobic surfaces thereby provide the basis for a possible mechanism by which the fungus can develop appropriately at the infection interface.
Figures



Similar articles
-
Backbone and sidechain (1)H, (13)C and (15)N chemical shift assignments of the hydrophobin MPG1 from the rice blast fungus Magnaporthe oryzae.Biomol NMR Assign. 2013 Apr;7(1):109-12. doi: 10.1007/s12104-012-9394-x. Epub 2012 May 19. Biomol NMR Assign. 2013. PMID: 22610311
-
Regulation of the MPG1 hydrophobin gene in the rice blast fungus Magnaporthe grisea.Mol Plant Microbe Interact. 2002 Dec;15(12):1253-67. doi: 10.1094/MPMI.2002.15.12.1253. Mol Plant Microbe Interact. 2002. PMID: 12481998
-
Identification and characterization of MPG1, a gene involved in pathogenicity from the rice blast fungus Magnaporthe grisea.Plant Cell. 1993 Nov;5(11):1575-90. doi: 10.1105/tpc.5.11.1575. Plant Cell. 1993. PMID: 8312740 Free PMC article.
-
Roles of Peroxisomes in the Rice Blast Fungus.Biomed Res Int. 2016;2016:9343417. doi: 10.1155/2016/9343417. Epub 2016 Aug 16. Biomed Res Int. 2016. PMID: 27610388 Free PMC article. Review.
-
Fungal Hydrophobins and Their Self-Assembly into Functional Nanomaterials.Adv Exp Med Biol. 2019;1174:161-185. doi: 10.1007/978-981-13-9791-2_5. Adv Exp Med Biol. 2019. PMID: 31713199 Review.
Cited by
-
In silico discovery of biomarkers for the accurate and sensitive detection of Fusarium solani.Front Bioinform. 2022 Sep 30;2:972529. doi: 10.3389/fbinf.2022.972529. eCollection 2022. Front Bioinform. 2022. PMID: 36304265 Free PMC article.
-
Recent Advances in Effector Research of Magnaporthe oryzae.Biomolecules. 2023 Nov 14;13(11):1650. doi: 10.3390/biom13111650. Biomolecules. 2023. PMID: 38002332 Free PMC article. Review.
-
Adsorption Kinetics and Self-Assembled Structures of Aspergillus oryzae Hydrophobin RolA on Hydrophobic and Charged Solid Surfaces.Appl Environ Microbiol. 2022 Mar 22;88(6):e0208721. doi: 10.1128/AEM.02087-21. Epub 2022 Feb 2. Appl Environ Microbiol. 2022. PMID: 35108098 Free PMC article.
-
CsPOM1, a DYRK Family Kinase, Plays Diverse Roles in Fungal Development, Virulence, and Stress Tolerance in the Anthracnose Pathogen Colletotrichum scovillei.Front Cell Infect Microbiol. 2022 Apr 26;12:861915. doi: 10.3389/fcimb.2022.861915. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35558103 Free PMC article.
-
α-Synuclein Aggregation Monitored by Thioflavin T Fluorescence Assay.Bio Protoc. 2018 Jul 20;8(14):e2941. doi: 10.21769/BioProtoc.2941. Bio Protoc. 2018. PMID: 30069495 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

