Disruption of a cystine transporter downregulates expression of genes involved in sulfur regulation and cellular respiration
- PMID: 27142334
- PMCID: PMC4920189
- DOI: 10.1242/bio.017517
Disruption of a cystine transporter downregulates expression of genes involved in sulfur regulation and cellular respiration
Abstract
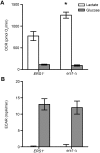
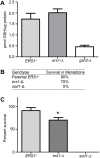
Cystine and cysteine are important molecules for pathways such as redox signaling and regulation, and thus identifying cellular deficits upon deletion of the Saccharomyces cerevisiae cystine transporter Ers1p allows for a further understanding of cystine homeostasis. Previous complementation studies using the human ortholog suggest yeast Ers1p is a cystine transporter. Human CTNS encodes the protein Cystinosin, a cystine transporter that is embedded in the lysosomal membrane and facilitates the export of cystine from the lysosome. When CTNS is mutated, cystine transport is disrupted, leading to cystine accumulation, the diagnostic hallmark of the lysosomal storage disorder cystinosis. Here, we provide biochemical evidence for Ers1p-dependent cystine transport. However, the accumulation of intracellular cystine is not observed when the ERS1 gene is deleted from ers1-Δ yeast, supporting the existence of modifier genes that provide a mechanism in ers1-Δ yeast that prevents or corrects cystine accumulation. Upon comparison of the transcriptomes of isogenic ERS1+ and ers1-Δ strains of S. cerevisiae by DNA microarray followed by targeted qPCR, sixteen genes were identified as being differentially expressed between the two genotypes. Genes that encode proteins functioning in sulfur regulation, cellular respiration, and general transport were enriched in our screen, demonstrating pleiotropic effects of ers1-Δ. These results give insight into yeast cystine regulation and the multiple, seemingly distal, pathways that involve proper cystine recycling.
Keywords: CTNS; Cystine; ERS1.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures

Similar articles
-
ERS1 encodes a functional homologue of the human lysosomal cystine transporter.FEBS J. 2005 May;272(10):2497-511. doi: 10.1111/j.1742-4658.2005.04670.x. FEBS J. 2005. PMID: 15885099
-
Cystinosin, the protein defective in cystinosis, is a H(+)-driven lysosomal cystine transporter.EMBO J. 2001 Nov 1;20(21):5940-9. doi: 10.1093/emboj/20.21.5940. EMBO J. 2001. PMID: 11689434 Free PMC article.
-
A Genetic Screen for Investigating the Human Lysosomal CystineTransporter, Cystinosin.Sci Rep. 2018 Feb 21;8(1):3442. doi: 10.1038/s41598-018-21483-x. Sci Rep. 2018. PMID: 29467429 Free PMC article.
-
The Role of Cystinosin in the Intermediary Thiol Metabolism and Redox Homeostasis in Kidney Proximal Tubular Cells.Antioxidants (Basel). 2018 Dec 3;7(12):179. doi: 10.3390/antiox7120179. Antioxidants (Basel). 2018. PMID: 30513914 Free PMC article. Review.
-
[From gene to disease: cystinosis].Ned Tijdschr Geneeskd. 2004 Mar 6;148(10):476-8. Ned Tijdschr Geneeskd. 2004. PMID: 15042893 Review. Dutch.
Cited by
-
Vacuolar hydrolysis and efflux: current knowledge and unanswered questions.Autophagy. 2019 Feb;15(2):212-227. doi: 10.1080/15548627.2018.1545821. Epub 2018 Nov 22. Autophagy. 2019. PMID: 30422029 Free PMC article. Review.
-
Exacerbating and reversing lysosomal storage diseases: from yeast to humans.Microb Cell. 2017 Aug 25;4(9):278-293. doi: 10.15698/mic2017.09.588. Microb Cell. 2017. PMID: 28913343 Free PMC article. Review.
-
Cystinosin is involved in Na+/H+ Exchanger 3 trafficking in the proximal tubular cells: new insights in the renal Fanconi syndrome in cystinosis.bioRxiv [Preprint]. 2025 Feb 12:2025.02.12.637793. doi: 10.1101/2025.02.12.637793. bioRxiv. 2025. PMID: 39990449 Free PMC article. Preprint.
-
Regulation of Amino Acid Transport in Saccharomyces cerevisiae.Microbiol Mol Biol Rev. 2019 Oct 16;83(4):e00024-19. doi: 10.1128/MMBR.00024-19. Print 2019 Nov 20. Microbiol Mol Biol Rev. 2019. PMID: 31619504 Free PMC article. Review.
-
In Vitro and In Vivo Models to Study Nephropathic Cystinosis.Cells. 2021 Dec 21;11(1):6. doi: 10.3390/cells11010006. Cells. 2021. PMID: 35011573 Free PMC article. Review.
References
-
- Adams A., Gottschling D. E., Kaiser C. A. and Stearns T. (1997). Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press.
-
- Boer V. M., de Winde J. H., Pronk J. T. and Piper M. D. W. (2003). The genome-wide transcriptional responses of Saccharomyces cerevisiae grown on glucose in aerobic chemostat cultures limited for carbon, nitrogen, phosphorus, or sulfur. J. Biol. Chem. 278, 3265-3274. 10.1074/jbc.M209759200 - DOI - PubMed
-
- Caro L. H. P., Tettelin H., Vossen J. H., Ram A. F. J., van den Ende H. and Klis F. M. (1997). In silicio identification of glycosyl-phosphatidylinositol-anchored plasma-membrane and cell wall proteins of Saccharomyces cerevisiae. Yeast 13, 1477-1489. 10.1002/(SICI)1097-0061(199712)13:15<1477::AID-YEA184>3.0.CO;2-L - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

