Patient-derived xenografts faithfully replicated clinical outcome in a phase II co-clinical trial of arsenic trioxide in relapsed small cell lung cancer
- PMID: 27142472
- PMCID: PMC4855771
- DOI: 10.1186/s12967-016-0861-5
Patient-derived xenografts faithfully replicated clinical outcome in a phase II co-clinical trial of arsenic trioxide in relapsed small cell lung cancer
Abstract
Background: SCLC has limited treatment options and inadequate preclinical models. Promising activity of arsenic trioxide (ASO) recorded in conventional preclinical models of SCLC supported the clinical evaluation of ASO in patients. We assessed the efficacy of ASO in relapsed SCLC patients and in corresponding patient-derived xenografts (PDX).
Methods: Single arm, Simon 2-stage, phase II trial to enroll patients with relapsed SCLC who have failed at least one line of therapy. ASO was administered as an intravenous infusion over 1-2 h daily for 4 days in week 1 and for 2 days in weeks 2-6 of an 8-week cycle. Treatment continued until disease progression. Pretreatment tumor biopsy was employed for PDX generation through direct implantation into subcutaneous pockets of SCID mice without in vitro manipulation and serially propagated for five generations. Ex vivo efficacy of cisplatin (3 mg/kg i.p. weekly) and ASO (3.75 mg/kg i.p. every other day) was tested in PDX representative of platinum sensitive and platinum refractory SCLC.
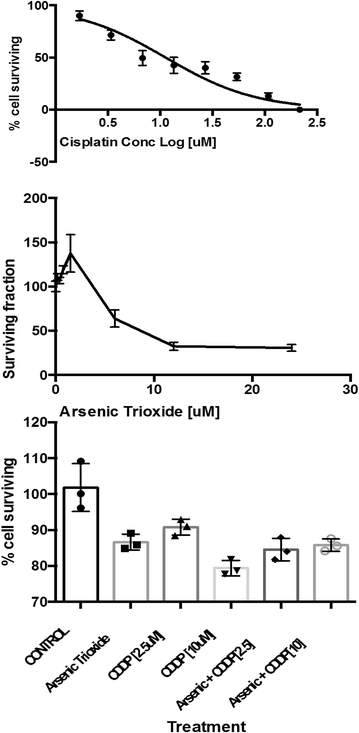
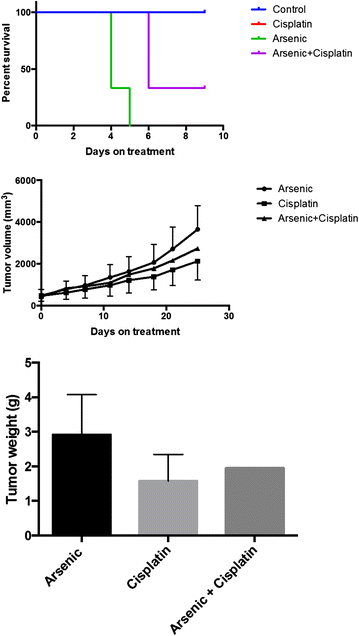
Results: The best response in 17 evaluable patients was stable disease in 2 (12 %), progressive disease in 15 (88 %) patients and median time-to-progression of seven (range 1-7) weeks. PDX was successfully grown in 5 of 9 (56 %) transplanted biopsy samples. Serially-propagated PDXs preserved characteristic small cell histology and genomic stability confirmed by immunohistochemistry, short tandem repeat (STR) profiling and targeted sequencing. ASO showed in vitro cytotoxicity but lacked in vivo efficacy against SCLC PDX tumor growth.
Conclusions: Cisplatin inhibited growth of PDX derived from platinum-sensitive SCLC but was ineffective against PDX from platinum-refractory SCLC. Strong concordance between clinical and ex vivo effects of ASO and cisplatin in SCLC supports the use of PDX models to prescreen promising anticancer agents prior to clinical testing in SCLC patients. Trial Registration The study was registered at http://www.clinicaltrials.gov (NCT01470248).
Keywords: Arsenic trioxide; Clinical trial; Efficacy; Ex vivo; Patient-derived xenograft; Small cell lung cancer; Survival.
Figures



Similar articles
-
[Establishment of A Patient-derived Xenotransplantation Animal Model for Small Cell Lung Cancer and Drug Resistance Model].Zhongguo Fei Ai Za Zhi. 2019 Jan 20;22(1):6-14. doi: 10.3779/j.issn.1009-3419.2019.01.03. Zhongguo Fei Ai Za Zhi. 2019. PMID: 30674387 Free PMC article. Chinese.
-
Combination Olaparib and Temozolomide in Relapsed Small-Cell Lung Cancer.Cancer Discov. 2019 Oct;9(10):1372-1387. doi: 10.1158/2159-8290.CD-19-0582. Epub 2019 Aug 15. Cancer Discov. 2019. PMID: 31416802 Free PMC article. Clinical Trial.
-
Combination of arsenic trioxide and chemotherapy in small cell lung cancer.Lung Cancer. 2013 Nov;82(2):222-30. doi: 10.1016/j.lungcan.2013.08.022. Epub 2013 Sep 3. Lung Cancer. 2013. PMID: 24041618
-
Topotecan: a review of its efficacy in small cell lung cancer.Drugs. 1999 Sep;58(3):533-51. doi: 10.2165/00003495-199958030-00020. Drugs. 1999. PMID: 10493279 Review.
-
Advances in pharmacotherapy of small cell lung cancer.Expert Opin Pharmacother. 2014 Nov;15(16):2385-96. doi: 10.1517/14656566.2014.957180. Epub 2014 Sep 26. Expert Opin Pharmacother. 2014. PMID: 25255939 Review.
Cited by
-
Co-clinical Trial of Novel Bispecific Anti-HER2 Antibody Zanidatamab in Patient-Derived Xenografts.Cancer Discov. 2024 May 1;14(5):828-845. doi: 10.1158/2159-8290.CD-23-0838. Cancer Discov. 2024. PMID: 38358339 Free PMC article. Clinical Trial.
-
Hsp90B enhances MAST1-mediated cisplatin resistance by protecting MAST1 from proteosomal degradation.J Clin Invest. 2019 Oct 1;129(10):4110-4123. doi: 10.1172/JCI125963. J Clin Invest. 2019. PMID: 31449053 Free PMC article.
-
[Advances in Patient Derived Tumor Xenograft (PDTX) Model from Lung Cancer].Zhongguo Fei Ai Za Zhi. 2017 Oct 20;20(10):715-719. doi: 10.3779/j.issn.1009-3419.2017.10.09. Zhongguo Fei Ai Za Zhi. 2017. PMID: 29061220 Free PMC article. Review. Chinese.
-
Genomic evolution of cancer models: perils and opportunities.Nat Rev Cancer. 2019 Feb;19(2):97-109. doi: 10.1038/s41568-018-0095-3. Nat Rev Cancer. 2019. PMID: 30578414 Free PMC article. Review.
-
The Generation and Application of Patient-Derived Xenograft Model for Cancer Research.Cancer Res Treat. 2018 Jan;50(1):1-10. doi: 10.4143/crt.2017.307. Epub 2017 Sep 13. Cancer Res Treat. 2018. PMID: 28903551 Free PMC article. Review.
References
-
- Ettinger DS, Jotte R, Lorigan P, Gupta V, Garbo L, Alemany C, Conkling P, Spigel DR, Dudek AZ, Shah C, et al. Phase II study of amrubicin as second-line therapy in patients with platinum-refractory small-cell lung cancer. J Clin Oncol. 2010;28:2598–2603. doi: 10.1200/JCO.2009.26.7682. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

