Transcriptome analysis indicated that Salmonella lipopolysaccharide-induced thymocyte death and thymic atrophy were related to TLR4-FOS/JUN pathway in chicks
- PMID: 27142675
- PMCID: PMC4855877
- DOI: 10.1186/s12864-016-2674-6
Transcriptome analysis indicated that Salmonella lipopolysaccharide-induced thymocyte death and thymic atrophy were related to TLR4-FOS/JUN pathway in chicks
Abstract
Background: Thymus is the crucial site for T cell development and once believed to be immune privileged. Recently, thymus has gained special attention as it is commonly targeted by infectious agents which may cause pathogenic tolerance and subsequent immunosuppression.
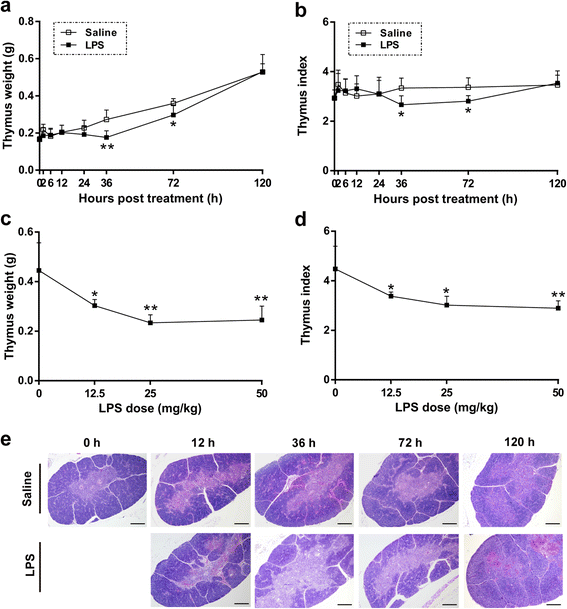
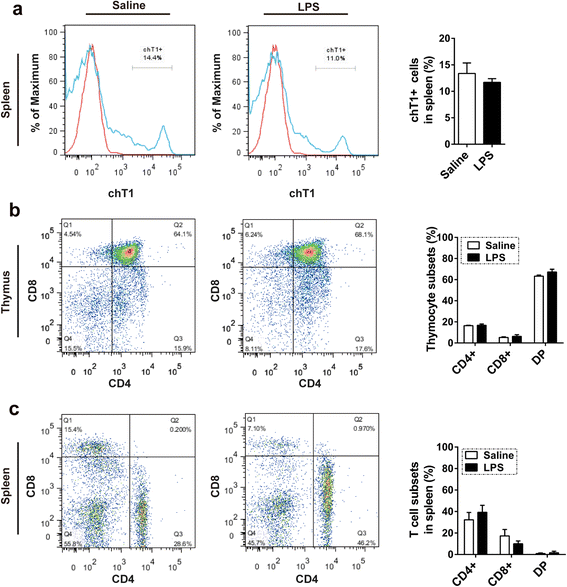
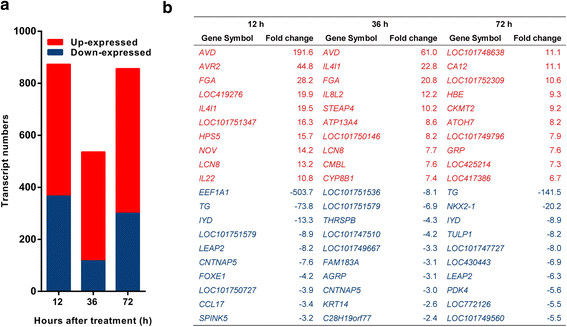
Results: We analyzed thymic responses to the challenge with Salmonella typhimurium (STm) or lipopolysaccharide (LPS) derived from STm in chicks. Newly hatched chicks were injected intraperitoneally with 5 × 10(4) CFU/mL STm or 50 mg/kg LPS. After LPS treatment, maximum thymocyte death (3 ~ 5-fold change) compared to controls was found at 12 h, and maximum loss of thymic weight (35 %) and reduced thymic index (20 %) were found at 36 h. After STm infection, maximum thymocyte death and thymic atrophy occurred at 36 and 72 h, respectively. No significant changes of thymic structure, chT1+ and CD4+/CD8+ T cell ratio were observed in thymus or spleen tissues after LPS treatment. Furthermore, transcriptome analysis revealed important roles for the TLR4-FOS/JUN signaling pathway in thymic injury. Thus, the major process of thymic atrophy in this study first involved activation of transcriptional factors FOS/JUN upon LPS binding to TLR4 that caused release of inflammatory factors, thereby inducing inflammatory responses and DNA damage and ultimately cell cycle arrest and thymic injury.
Conclusions: STm and Salmonella LPS could induce acute chick thymic injury. LPS treatment acted faster than STm. TLR4-FOS/JUN pathway may play an important role in LPS induced chick thymic injury.
Keywords: Salmonella typhimurium; Thymus injury; Transcriptome; chT1.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

