The effect of providing feedback on inhaler technique and adherence from an electronic audio recording device, INCA®, in a community pharmacy setting: study protocol for a randomised controlled trial
- PMID: 27142873
- PMCID: PMC4855368
- DOI: 10.1186/s13063-016-1362-9
The effect of providing feedback on inhaler technique and adherence from an electronic audio recording device, INCA®, in a community pharmacy setting: study protocol for a randomised controlled trial
Abstract
Background: Poor adherence to inhaled medication may lead to inadequate symptom control in patients with respiratory disease. In practice it can be difficult to identify poor adherence. We designed an acoustic recording device, the INCA® (INhaler Compliance Assessment) device, which, when attached to an inhaler, identifies and records the time and technique of inhaler use, thereby providing objective longitudinal data on an individual's adherence to inhaled medication. This study will test the hypothesis that providing objective, personalised, visual feedback on adherence to patients in combination with a tailored educational intervention in a community pharmacy setting, improves adherence more effectively than education alone.
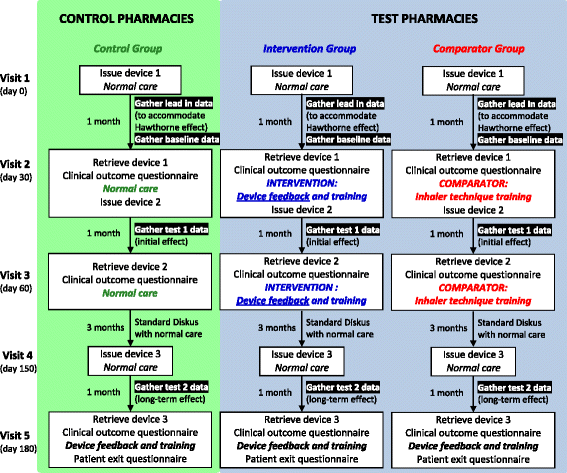
Methods/design: The study is a prospective, cluster randomised, parallel-group, multi-site study conducted over 6 months. The study is designed to compare current best practice in care (i.e. routine inhaler technique training) with the use of the INCA® device for respiratory patients in a community pharmacy setting. Pharmacies are the unit of randomisation and on enrolment to the study they will be allocated by the lead researcher to one of the three study groups (intervention, comparator or control groups) using a computer-generated list of random numbers. Given the nature of the intervention neither pharmacists nor participants can be blinded. The intervention group will receive feedback from the acoustic recording device on inhaler technique and adherence three times over a 6-month period along with inhaler technique training at each of these times. The comparator group will also receive training in inhaler use three times over the 6-month study period but no feedback on their habitual performance. The control group will receive usual care (i.e. the safe supply of medicines and advice on their use). The primary outcome is the rate of participant adherence to their inhaled medication, defined as the proportion of correctly taken doses of medication at the correct time relative to the prescribed interval. Secondary outcomes include exacerbation rates and quality of life measures. Differences in the timing and technique of inhaler use as altered by the interventions will also be assessed. Data will be analysed on an intention-to-treat and a per-protocol basis. Sample size has been calculated with reference to comparisons to be made between the intervention and comparator clusters and indicates 75 participants per cluster. With an estimated 10 % loss to follow-up we will be able to show a 20 % difference between the population means of the intervention and comparator groups with a power of 0.8. The Type I error probability associated with the test of the null hypothesis is 0.05.
Discussion: This clinical trial will establish whether providing personalised feedback to individuals on their inhaler use improves adherence. It may also be possible to enhance the role of pharmacists in clinical care by identifying patients in whom alteration of either therapy or inhaler device is appropriate.
Registration: ClinicalTrials.gov NCT02203266 .
Keywords: Adherence; Cluster randomised trial; Community pharmacy; INCA® electronic monitor; Inhaler technique; Patient education.
Figures
References
-
- World Health Organisation. The top 10 causes of death in the world, 2000 and 2012. WHO Fact Sheet Number 310. Accessed via http://www.who.int/mediacentre/factsheets/fs310/en/ on 8 September 2014.
-
- Global Initiative for Asthma. Global strategy for asthma management and prevention 2014. Accessed via www.ginasthma.org on 8 September 2014.
-
- Global Initiative for Chronic Obstructive Pulmonary Disease. Global strategy for the diagnosis, management and prevention of COPD 2014. Accessed via http://www.goldcopd.org/ on 8 September 2014.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical