Automated synthesis of [(18)F]DCFPyL via direct radiofluorination and validation in preclinical prostate cancer models
- PMID: 27142881
- PMCID: PMC4854855
- DOI: 10.1186/s13550-016-0195-6
Automated synthesis of [(18)F]DCFPyL via direct radiofluorination and validation in preclinical prostate cancer models
Abstract
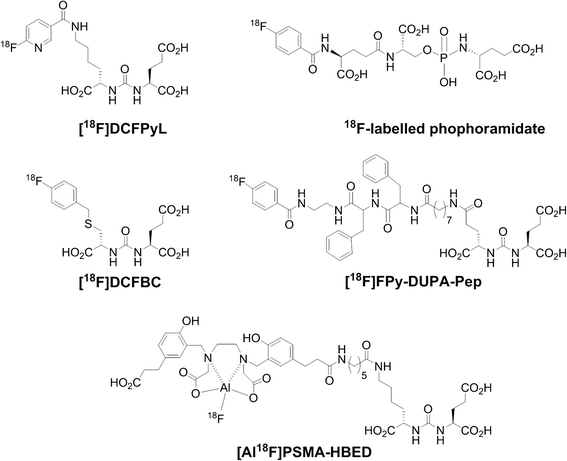
Background: Prostate-specific membrane antigen (PSMA) is frequently overexpressed and upregulated in prostate cancer. To date, various (18)F- and (68)Ga-labeled urea-based radiotracers for PET imaging of PSMA have been developed and entered clinical trials. Here, we describe an automated synthesis of [(18)F]DCFPyL via direct radiofluorination and validation in preclinical models of prostate cancer.
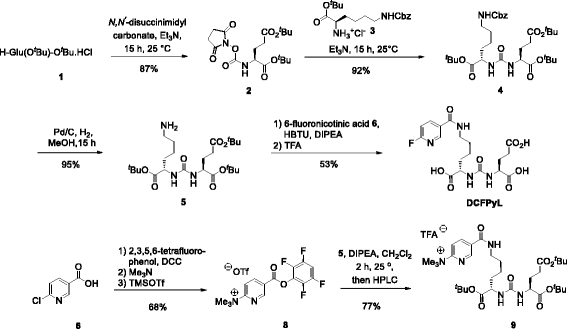
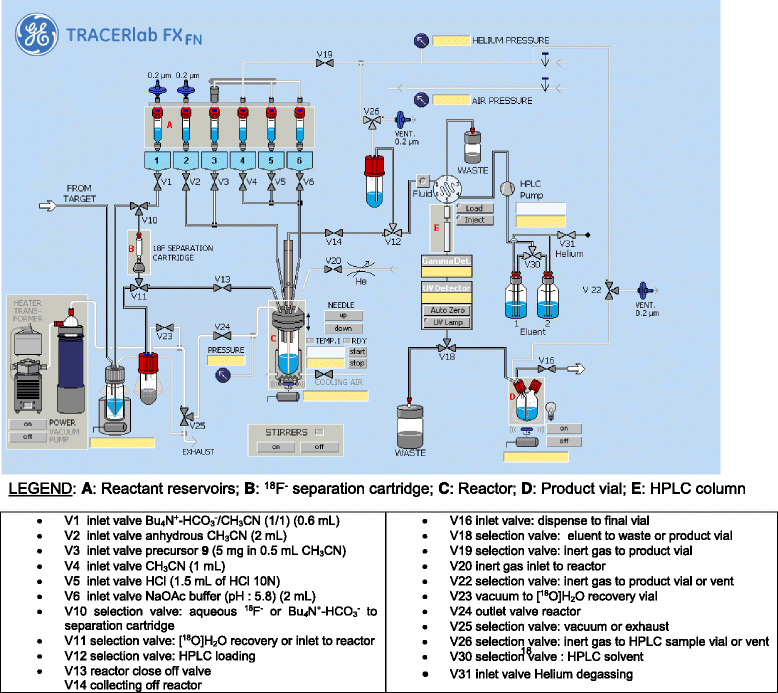
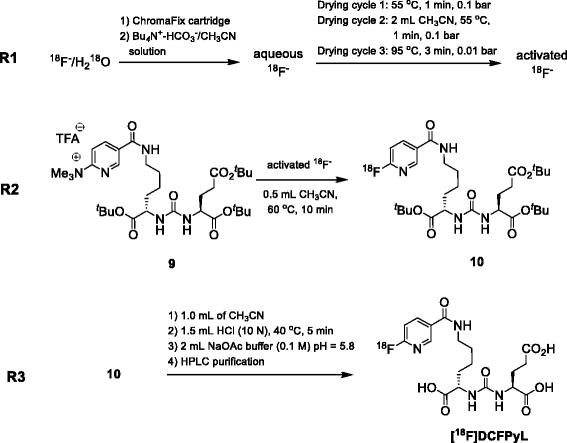
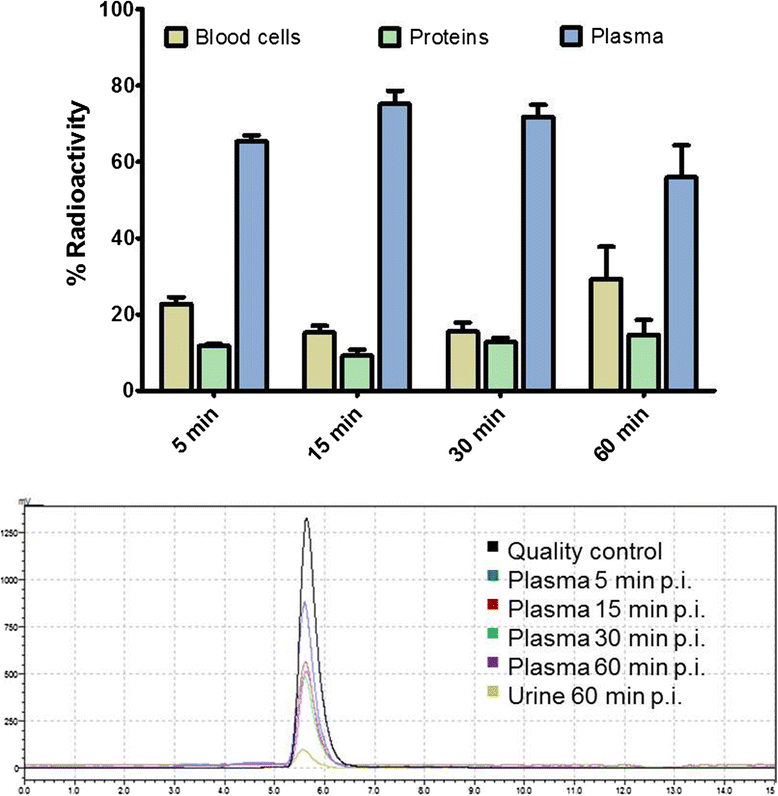
Methods: [(18)F]DCFPyL was synthesized via direct nucleophilic heteroaromatic substitution reaction in a single reactor TRACERlab FXFN automated synthesis unit. Radiopharmacological evaluation of [(18)F]DCFPyL involved internalization experiments, dynamic PET imaging in LNCaP (PSMA+) and PC3 (PSMA-) tumor-bearing BALB/c nude mice, biodistribution studies, and metabolic profiling. In addition, reversible two-tissue compartmental model analysis was used to quantify pharmacokinetics of [(18)F]DCFPyL in LNCaP and PC3 tumor models.
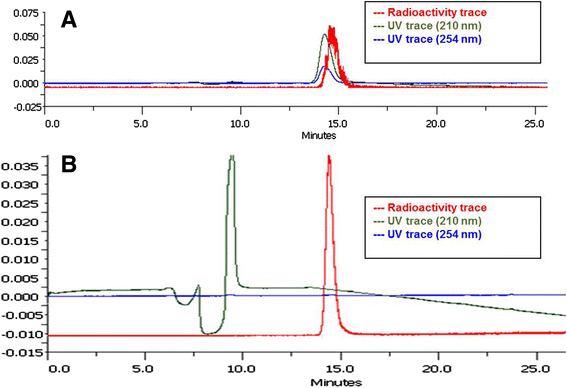
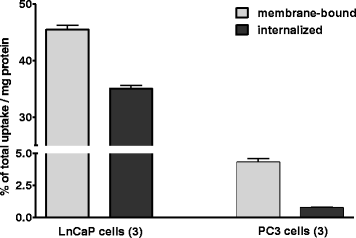
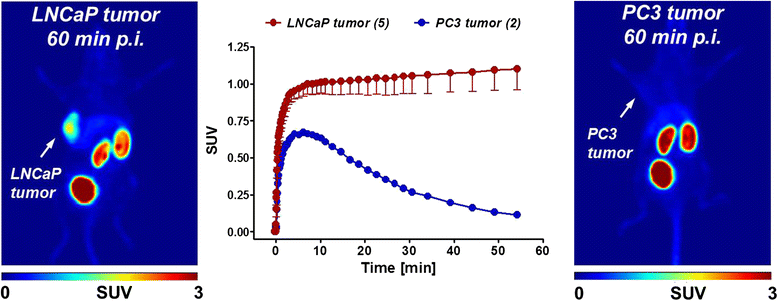
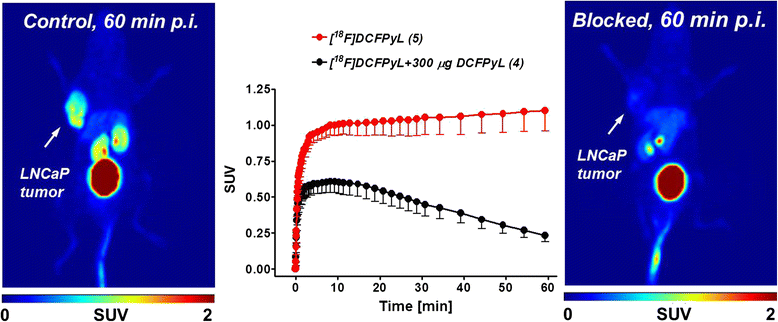
Results: Automated radiosynthesis afforded radiotracer [(18)F]DCFPyL in decay-corrected radiochemical yields of 23 ± 5 % (n = 10) within 55 min, including HPLC purification. Dynamic PET analysis revealed rapid and high uptake of radioactivity (SUV5min 0.95) in LNCaP tumors which increased over time (SUV60min 1.1). Radioactivity uptake in LNCaP tumors was blocked in the presence of nonradioactive DCFPyL (SUV60min 0.22). The muscle as reference tissue showed rapid and continuous clearance over time (SUV60min 0.06). Fast blood clearance of radioactivity resulted in tumor-blood ratios of 1.0 after 10 min and 8.3 after 60 min. PC3 tumors also showed continuous clearance of radioactivity over time (SUV60min 0.11). Kinetic analysis of PET data revealed the two-tissue compartmental model as best fit with K 1 = 0.12, k 2 = 0.18, k 3 = 0.08, and k 4 = 0.004 min(-1), confirming molecular trapping of [(18)F]DCFPyL in PSMA+ LNCaP cells.
Conclusions: [(18)F]DCFPyL can be prepared for clinical applications simply and in good radiochemical yields via a direct radiofluorination synthesis route in a single reactor automated synthesis unit. Radiopharmacological evaluation of [(18)F]DCFPyL confirmed high PSMA-mediated tumor uptake combined with superior clearance parameters. Compartmental model analysis points to a two-step molecular trapping mechanism based on PSMA binding and subsequent internalization leading to retention of radioactivity in PSMA+ LNCaP tumors.
Keywords: 18FDCFPyL; Automated radiosynthesis; Positron emission tomography (PET); Prostate cancer; Prostate-specific membrane antigen (PSMA).
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

