Matrix stiffness promotes cartilage endplate chondrocyte calcification in disc degeneration via miR-20a targeting ANKH expression
- PMID: 27142968
- PMCID: PMC4855171
- DOI: 10.1038/srep25401
Matrix stiffness promotes cartilage endplate chondrocyte calcification in disc degeneration via miR-20a targeting ANKH expression
Abstract
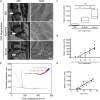
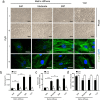
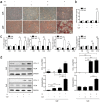
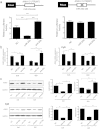
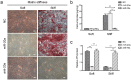
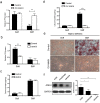
The mechanical environment is crucial for intervertebral disc degeneration (IDD). However, the mechanisms underlying the regulation of cartilage endplate (CEP) calcification by altered matrix stiffness remain unclear. In this study, we found that matrix stiffness of CEP was positively correlated with the degree of IDD, and stiff matrix, which mimicked the severe degeneration of CEP, promoted inorganic phosphate-induced calcification in CEP chondrocytes. Co-expression analysis of the miRNA and mRNA profiles showed that increasing stiffness resulted in up-regulation of miR-20a and down-regulation of decreased ankylosis protein homolog (ANKH) during inorganic phosphate-induced calcification in CEP chondrocytes. Through a dual luciferase reporter assay, we confirmed that miR-20a directly targets 3'-untranslated regions of ANKH. The inhibition of miR-20a attenuated the calcium deposition and calcification-related gene expression, whereas the overexpression of miR-20a enhanced calcification in CEP chondrocytes on stiff matrix. The rescue of ANKH expression restored the decreased pyrophosphate efflux and inhibited calcification. In clinical samples, the levels of ANKH expression were inversely associated with the degeneration degree of CEP. Thus, our findings demonstrate that the miR-20a/ANKH axis mediates the stiff matrix- promoted CEP calcification, suggesting that miR-20a and ANKH are potential targets in restraining the progression of IDD.
Figures
Similar articles
-
LTF ameliorates cartilage endplate degeneration by suppressing calcification, senescence and matrix degradation through the JAK2/STAT3 pathway.J Cell Mol Med. 2024 Oct;28(19):e18267. doi: 10.1111/jcmm.18267. J Cell Mol Med. 2024. PMID: 39392081 Free PMC article.
-
Human cartilaginous endplate degeneration is induced by calcium and the extracellular calcium-sensing receptor in the intervertebral disc.Eur Cell Mater. 2016 Jul 25;32:137-51. doi: 10.22203/ecm.v032a09. Eur Cell Mater. 2016. PMID: 27452962
-
MiR-34a promotes Fas-mediated cartilage endplate chondrocyte apoptosis by targeting Bcl-2.Mol Cell Biochem. 2015 Aug;406(1-2):21-30. doi: 10.1007/s11010-015-2420-4. Epub 2015 Apr 25. Mol Cell Biochem. 2015. PMID: 25910896
-
Research Progress on the Role of Cartilage Endplate in Intervertebral Disc Degeneration.Cell Biochem Funct. 2024 Sep;42(7):e4118. doi: 10.1002/cbf.4118. Cell Biochem Funct. 2024. PMID: 39267363 Review.
-
The role of ANKH in pathologic mineralization of cartilage.Curr Opin Rheumatol. 2016 Mar;28(2):145-51. doi: 10.1097/BOR.0000000000000247. Curr Opin Rheumatol. 2016. PMID: 26599446 Review.
Cited by
-
Insights into the mechanical microenvironment within the cartilaginous endplate: An emerging role in maintaining disc homeostasis and normal function.Heliyon. 2024 May 11;10(10):e31162. doi: 10.1016/j.heliyon.2024.e31162. eCollection 2024 May 30. Heliyon. 2024. PMID: 38803964 Free PMC article. Review.
-
[Effect of HBP-A on meniscal injury and pathological hypertrophy and calcification of the meniscus].Nan Fang Yi Ke Da Xue Xue Bao. 2016 Apr 20;37(4):431-437. doi: 10.3969/j.issn.1673-4254.2017.04.02. Nan Fang Yi Ke Da Xue Xue Bao. 2016. PMID: 28446392 Free PMC article. Chinese.
-
The potential role and trend of HIF‑1α in intervertebral disc degeneration: Friend or foe? (Review).Mol Med Rep. 2021 Apr;23(4):239. doi: 10.3892/mmr.2021.11878. Epub 2021 Feb 4. Mol Med Rep. 2021. PMID: 33537810 Free PMC article. Review.
-
MicroRNAs in Intervertebral Disc Degeneration, Apoptosis, Inflammation, and Mechanobiology.Int J Mol Sci. 2020 May 20;21(10):3601. doi: 10.3390/ijms21103601. Int J Mol Sci. 2020. PMID: 32443722 Free PMC article. Review.
-
The potential mechanisms and application prospects of non-coding RNAs in intervertebral disc degeneration.Front Endocrinol (Lausanne). 2022 Dec 8;13:1081185. doi: 10.3389/fendo.2022.1081185. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36568075 Free PMC article. Review.
References
-
- Borenstein D. G. Epidemiology, etiology, diagnostic evaluation, and treatment of low back pain. Current opinion in rheumatology 13, 128–134 (2001). - PubMed
-
- Kelsey J. L. & White A. A. 3rd. Epidemiology and impact of low-back pain. Spine (Phila Pa 1976) 5, 133–142 (1980). - PubMed
-
- Riihimaki H. Low-back pain, its origin and risk indicators. Scand J Work Environ Health 17, 81–90 (1991). - PubMed
-
- Urban J. P., Smith S. & Fairbank J. C. Nutrition of the intervertebral disc. Spine (Phila. Pa 1976) 29, 2700–2709 (2004). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

